【Research Background】
Metallic lithium is considered an ideal anode material in battery chemistry due to its high theoretical capacity (3860 mAh g -1) and low reduction potential (3.04 V vs. SHE). However, metallic lithium anodes exhibit high chemical reactivity, spontaneously forming a brittle and uneven solid electrolyte interphase (SEI) layer upon contact with organic electrolytes. The heterogeneity and poor mechanical/interface stability of the SEI layer lead to uneven lithium ion flux and continuous cracking/breaking of the interphase layer. Consequently, the phenomenon of uneven lithium deposition and the gradual increase in electrolyte consumption during the entire battery cycle result in low cycling efficiency. Particularly, these issues worsen dramatically during fast charging and high area capacity scenarios, often leading to internal short circuits under lithium dendrite impacts, posing serious safety risks. To date, scientists have made significant efforts to enhance the stability of the SEI layer, including optimizing electrolytes to promote the in-situ formation of stable SEI layers and constructing artificial SEI layers to replace the solid electrolyte interphase derived from the electrolyte and metallic lithium anode. Among these, optimizing electrolytes is challenging as the formed SEI layer requires continuous consumption of electrolytes, salt anions, or sacrificial additives in the electrolyte, making the composition and structure of the SEI layer difficult to control. In contrast, constructing artificial protective layers is a direct method to avoid adverse reactions and address the instability and fragility of the derived SEI layer.
【Work Introduction】
Recently, Professors Jiajun Fu and Tao Chen from Nanjing University of Science and Technology made significant progress in the field of metallic lithium anodes, publishing a research paper titled “Fatigue-Free and Skin-like Supramolecular Ion-Conductive Elastomeric Interphases for Stable Lithium Metal Batteries” in the internationally renowned journal ACS Nano. This article employs a fatigue-free, skin-like dynamic supramolecular ion-conductive elastomer (DSIEI) as a protective layer for metallic lithium anodes. The presence of the lock-phase molecular structure in the system enables long-term stable cycling of lithium metal batteries under high current density and large area capacity. The article is published in the top international journalACS Nano.
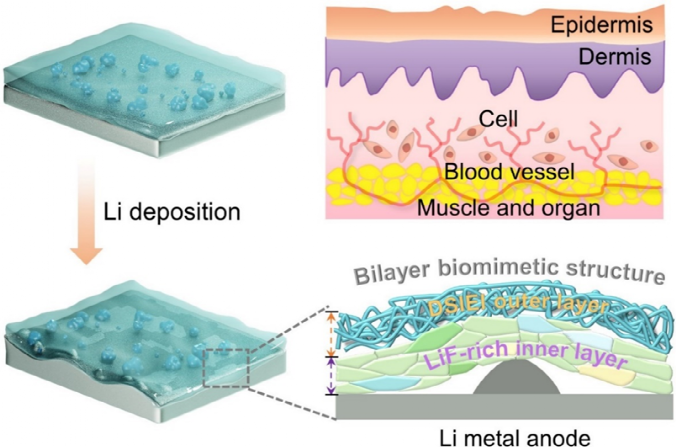
Figure 1. Schematic diagram and protection mechanism of DSIEI as a protective layer for lithium metal anodes.
【Content Description】
1. The multiple interactions present in DSIEI endow the material with excellent mechanical properties.
In the DSIEI system, strong and weak hydrogen bond synergistic effects exist between urea groups, between urethane groups, or between urea and urethane groups, collectively endowing the DSIEI material with excellent mechanical properties. After subjecting DSIEI samples to continuous stretching for 5 cycles (strain of 50%) followed by a 5-minute rest, the loading-unloading cycle curve of DSIEI overlaps almost completely with the first cycle curve, indicating that DSIEI possesses good and rapid elastic recovery ability without external stimuli. After experiencing maximum puncture displacement, the DSIEI film can still recover to its initial state after the needle tip stress is gradually released, demonstrating excellent puncture resistance.
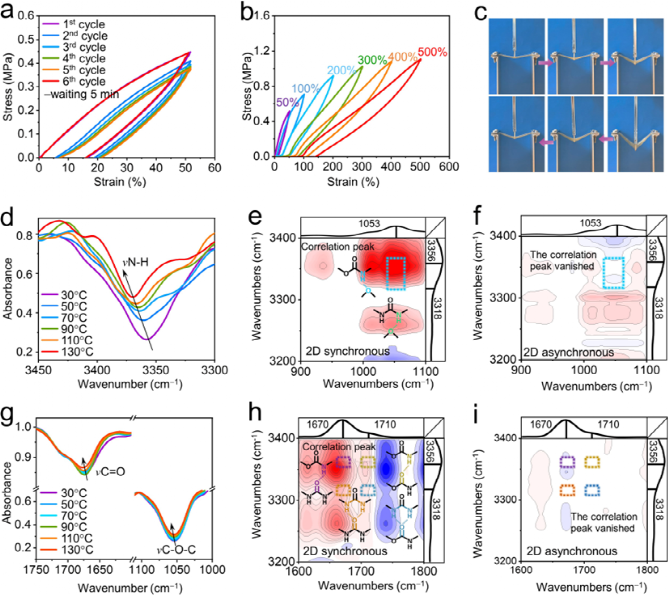
Figure 2. (a) Continuous cyclic stretching curve of DSIEI samples at 50% strain, (b) cyclic stretching curves of DSIEI samples at different strains, (c) photos of approximately 0.8 mm thick DSIEI film being punctured by a steel needle under different deformation states. (d) and (g) FTIR spectra of DSIEI at varying temperatures from 30 to 130°C. 2DCOS synchronous (e, h) and asynchronous (f, i) correlated FTIR spectra.
2. Understanding the lithium ion transport mechanism through spectral analysis, theoretical calculations, and molecular dynamics simulations.
The DSIEI-protected metallic lithium anode (DSIEI@Li) exhibits a wider electrochemical stability window and exchange current density. From the Raman spectrum, it can be observed that the proportion of free TFSI– increases with the addition of DSE, indicating that DSE facilitates the dissociation of Li+-TFSI– complexes and uniform transport of lithium ions. The coulombic efficiency and cycling stability of the DSIEI@Cu asymmetric battery are significantly improved compared to bare copper. Theoretical calculations indicate that the dissociation energies of DSE-LiTFSI on the PPG segment, urethane units, and urea units for Li+ and TFSI– are 4.94 eV, 4.36 eV, and 2.23 eV, respectively, suggesting that LiTFSI is more easily dissociated/decoupled in the urea region. The absorption energy between the urea region and Li+ is -3.35 eV, lower than the binding energies between Li+ and the PPG segment (-0.60 eV) and between the urethane units (-1.30 eV). Therefore, the coordination interactions between Li+-O in the PPG segment are relatively loose, and the lower dissociation energy facilitates rapid ion transport, leading to excellent ionic conductivity. This is also consistent with the results of molecular dynamics simulations.
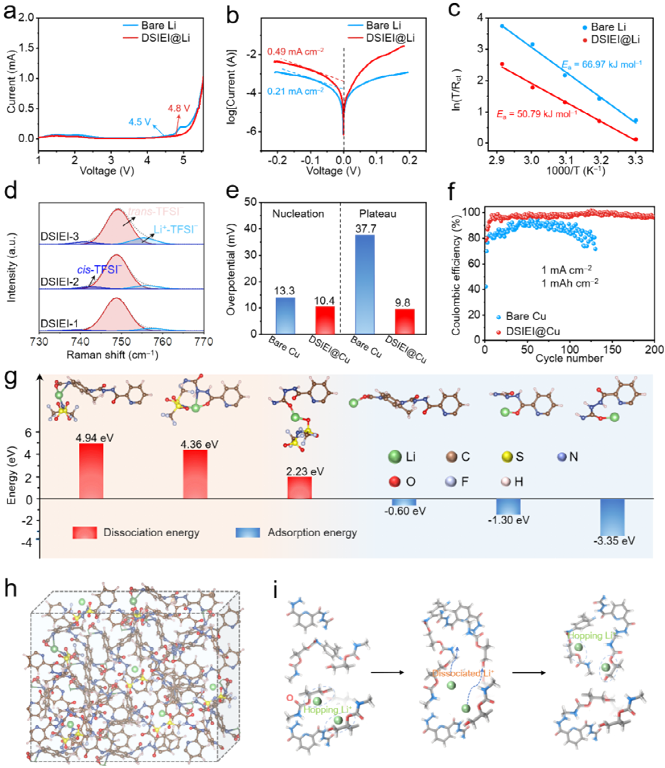
Figure 3. (a) LSV curves of bare lithium and DISEI@Li, (b) Tafel curves of DISEI@Li and bare lithium symmetric batteries, (c) Arrhenius fitting curves determining the activation energies of bare lithium and DSIEI@Li symmetric batteries, (d) Raman spectra of DISEI-1, DISEI-2, and DISEI-3, (e) nucleation overpotential and mass transfer control platform of bare Cu and DISEI@Cu, (f) coulombic efficiency of the asymmetric battery at a current density of 1 mA cm-2 with a deposition capacity of 1 mAh cm-2, (g) dissociation energy of DSE-LiTFSI complex and absorption energy between Li+ and oxygen-containing groups, (h) MD simulation model after equilibrium of the DSIEI system, (i) schematic diagram of Li+ migration with loose coordination in the PPG chain.
3. Long cycling and high capacity electrochemical cycling performance.
The symmetric battery with bare lithium anode shows significant voltage fluctuation in the deposition/stripping voltage distribution curve under a current density of 1 mAh cm-2 and area capacity of 1 mAh cm-2, with polarization voltage gradually increasing, leading to a short circuit within 300 hours. In contrast, the symmetric battery with DSIEI@Li anode demonstrates a very stable voltage platform over 4500 hours (over 6 months). As the current density increases to 5 mA cm-2 and area capacity reaches 5 mAh cm-2, the advantages of DSIEI@Li become even more pronounced. For the DSIEI@Li anode, over 1600 hours of stable and highly reversible lithium plating/stripping is observed, while the battery with bare lithium anode exhibits severe voltage oscillations from the initial stage (5 mA cm-2, 5 mAh cm-2). Furthermore, even at extremely high current densities and area capacities of 10 mA cm-2 and 10 mAh cm-2, the DSIEI@Li symmetric battery still shows at least 600 hours of cycling stability, significantly outperforming the bare lithium symmetric battery.
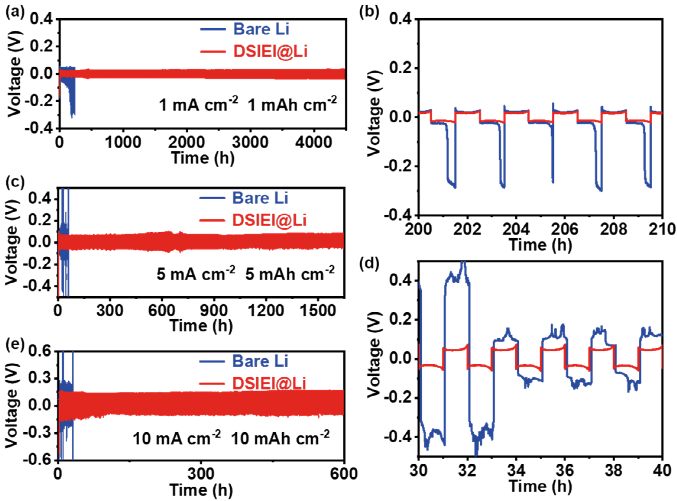
Figure 4. Cycling performance of symmetric batteries based on bare lithium and DSIEI@Li anodes. (a) 1 mA cm-2/1 mAh cm-2 and (b) corresponding enlarged view from 200-210 hours, (c) 5 mA cm-2/5 mAh cm-2 and (d) corresponding enlarged view from 30-40 hours, (e) voltage-time curve of symmetric batteries at 10 mA cm-2/10 mAh cm-2.
4. Observing morphology using SEM and in-situ observations.
After repeating deposition/stripping for 300 hours under a current density of 1 mA cm-2 and area capacity of 1 mAh cm-2, porous and whisker-like lithium dendrites were detected on the surface of the bare lithium electrode, severely hindering the transport of Li+ and leading to the accumulation of inactive lithium. Due to the accumulation of dendrites and inactive lithium, the thickness of the porous structure is approximately 9 μm. In contrast, the surface of the DSIEI@Li anode exhibits a smooth and dense lithium deposition morphology, with no significant lithium dendrite growth. The roughness of the SEI formed on the electrode was studied using electrochemical atomic force microscopy (AFM). The roughness of the DSIEI@Li electrode after cycling increased to about 3.0 µm, much lower than that of the cycled bare Li anode (approximately 11.8 µm). These results indicate that the DSIEI elastic interphase can effectively suppress lithium dendrite formation and mitigate volume changes during cycling.
In-situ optical microscopy observed the dynamic morphological evolution of lithium deposition on bare lithium and DSIEI@Li electrodes at a plating current density of 0.5 mA cm-2. Before lithium plating, both bare lithium and DSIEI@Li anodes exhibited smooth surfaces. However, at 15 minutes, significant uneven lithium deposition was observed on the surface of bare lithium, rapidly evolving into moss-like lithium clusters and continuously forming dendrites along the bare Li surface over the next 45 minutes. In stark contrast, throughout the entire electro-deposition process, the surface of the DSIEI@Li anode remained dense with no dendrite growth, further confirming the advantages of DSIEI in stabilizing the Li/electrolyte interface and suppressing dendrite growth.
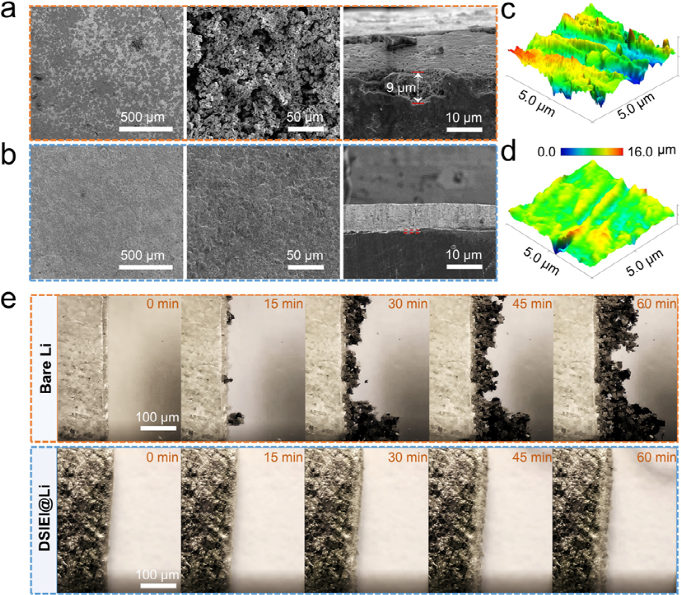
Figure 5. After 300 hours of cycling under conditions of 1mA cm-2, 1mAh cm-2, (a) scanning electron microscopy surface and cross-sectional images of bare lithium and (b) DSIEI@Li, (c) AFM characterization of surface roughness of bare lithium and (d) DSIEI@Li after 300 hours of cycling, (e) lithium deposition conditions of bare lithium and DSIEI@Li at current density of 0.5 mA cm−2 at 0, 15, 30, 45, and 60 minutes.
【Conclusion】
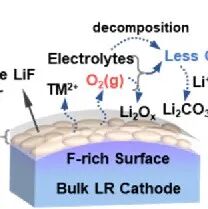
This study developed a fatigue-free, skin-like dynamic supramolecular ion-conductive elastomeric interface, providing theoretical guidance for achieving dendrite-free and high-efficiency lithium metal anodes. The loose Li+-O coordination interactions present in the lock-phase structure facilitate rapid lithium ion transport, while the supramolecular quadruple hydrogen bonds in the hard domains are responsible for mechanical enhancement, and weak hydrogen bonds can dissipate the strain energy caused by the repeated protrusion of lithium dendrites. This design endows the elastic interphase with unparalleled mechanical strength/toughness, energy dissipation capability, fatigue resistance, ionic conductivity, and high Li+ transference number. Additionally, the enrichment of TFSI anions within the DSIEI induces the formation of a LiF-rich inner layer, creating a biomimetic double-layer structure. Due to these ideal characteristics, the DSIEI@Li anode exhibits a long cycling life of over 600 hours under high current density and area capacity (10 mA cm-2, 10 mAh cm-2). Furthermore, under the constraints of limiting lithium excess and low N/P ratios, the DSIEI@Li full battery can operate stably in the presence of high-load cathodes. We hope that this design concept of dynamic supramolecular ion-conductive elastomeric interfaces can open new opportunities for the development of stable high-energy lithium metal batteries.
Po Hu, Wei Chen, Yang Wang, Tao Chen*, Xiaohu Qian, Wenqi Li, Jiaoyang Chen, and Jiajun Fu*, Fatigue-Free and Skin-like Supramolecular Ion-Conductive Elastomeric Interphases for Stable Lithium Metal Batteries, ACS Nano, 2023.
https://doi.org/10.1021/acsnano.3c06171
Is the large-scale application of sodium-ion batteries just around the corner or a long way off?
2023-08-10

One solution to the lead leakage problem in perovskite solar cells.
2023-08-10
Professor Liu Yongchang’s ACS Energy Lett.: New sodium-ion battery cathode Na3.5Fe0.5VCr0.5(PO4)3 with multi-electron reactions and low volume strain.
2023-08-10

Professor Yang Yong’s JMR review: The application of solid-state NMR technology in sodium-ion batteries.
2023-08-10

Shenzhen University team led by Tian Lei, Zhu Caizhen, and Xu Jian: High-performance solid-state polymer batteries constructed using in-situ hybrid crosslinking strategies.
2023-08-10
Professor Zhang Hulin’s team from Taiyuan University of Technology: Thermoelectric hydrogel patches for passive recognition of human outdoor sports.
2023-08-10
The future form of electrodes: The impact of dry powder morphology and manufacturing processes on electrode structure and performance.
2023-08-09
New composite single-ion conductive solid electrolytes: Adding bricks to the high-energy-density all-solid-state Li-S battery!
2023-08-09
Harbin Institute of Technology – Su Xin team and collaborators | AEnM review: The application prospects and mechanisms of bis(oxalic) lithium borate as a lithium battery electrolyte additive.
2023-08-09

National University of Defense Technology Energy Materials and Devices team Adv Energy Mater: Re-examining the importance of the CEI interface in lithium-rich cathodes.
2023-08-09