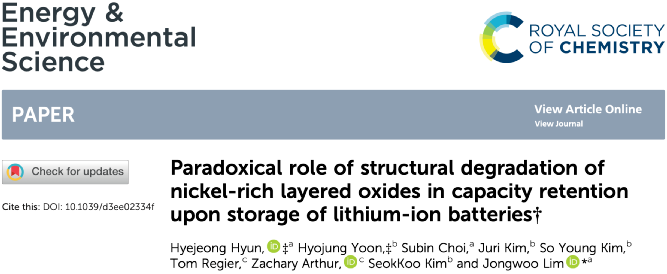
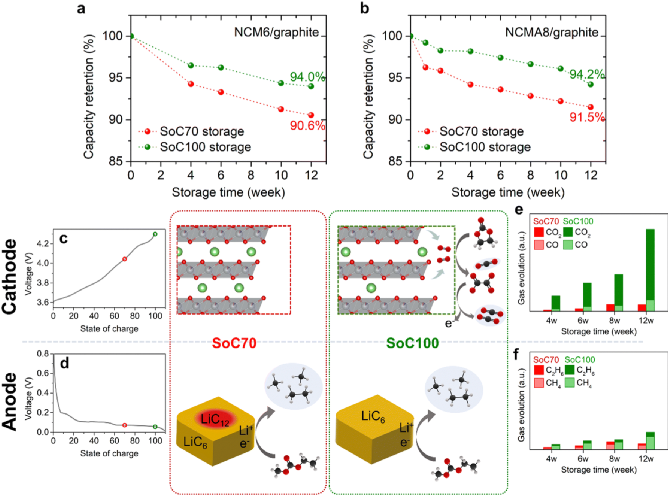


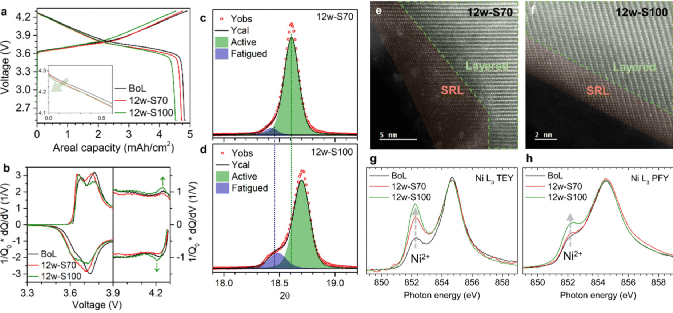
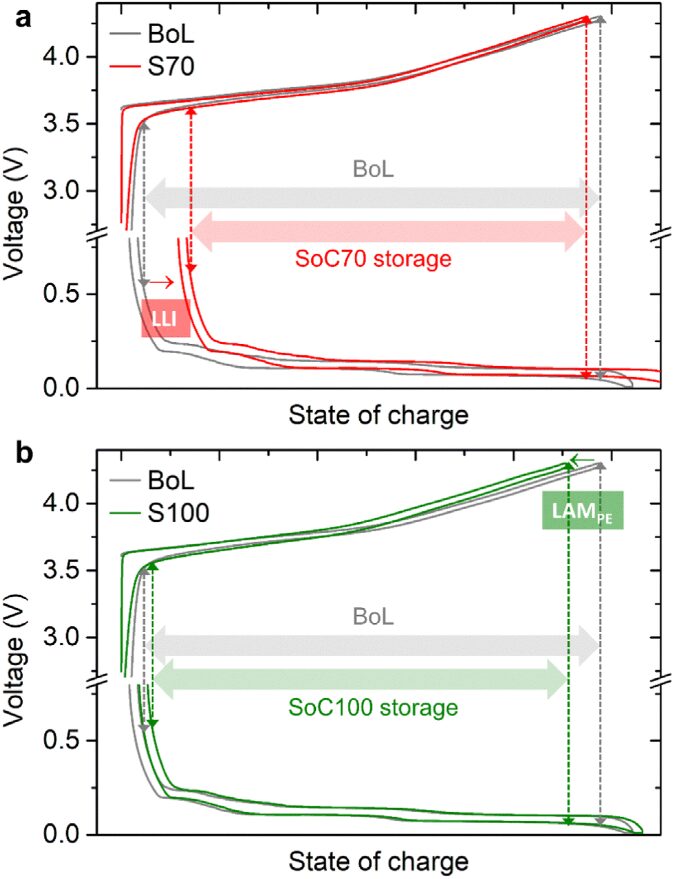
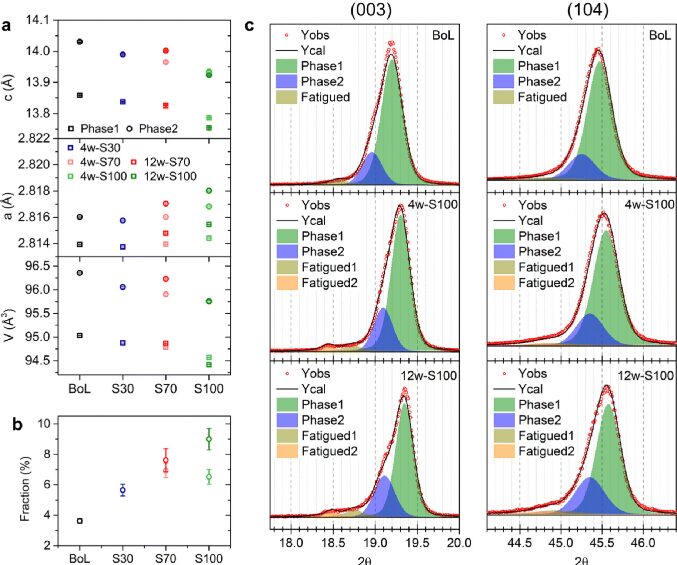
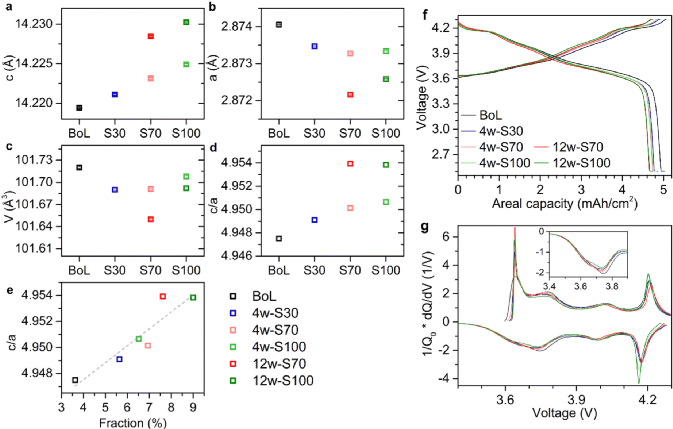
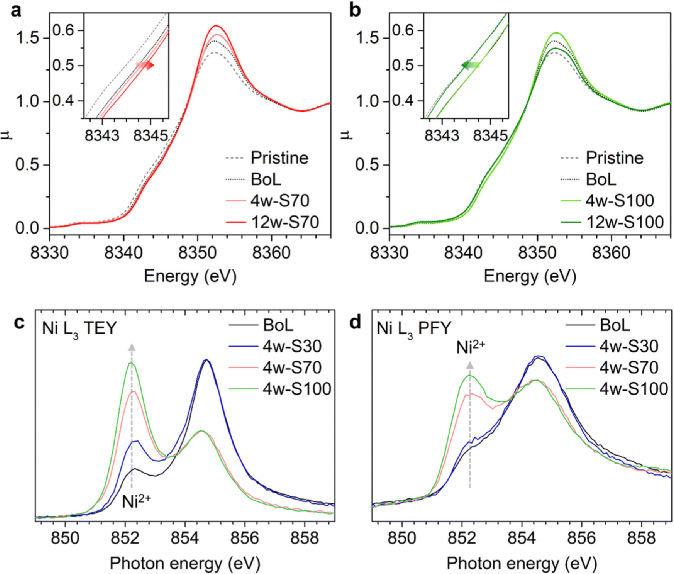
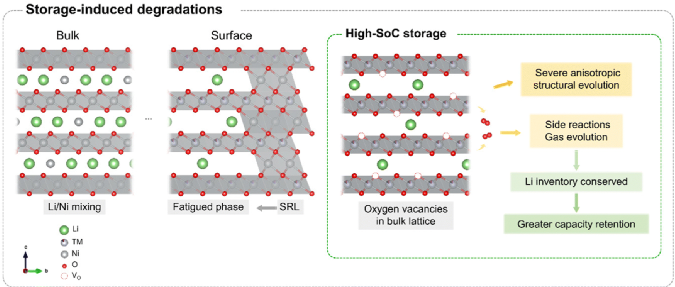
Research Group of Xin Huo Lin—Polymer Electrolytes: Single Ion Conduction (SIC) or Dual Ion Conduction (DIC)?
2023-08-11

Silicon-based Batteries—How to Achieve High Capacity and High Rate?
2023-08-11

Imperial College London’s Yunlong Zhao, University of Surrey’s Kai Yang, Peking University’s Feng Pan, etc. EES: High Reversible Lithium-Carbon Dioxide Batteries: From Development of On-Chip In Situ Characterization Test Platforms to Applications in Pouch Batteries
2023-08-11

Nanjing University of Science and Technology’s Professors Fu Jiajun and Chen Tao ACS Nano: Anti-Fatigue, Skin-like Supramolecular Ionic Conductive Elastic Layer Stabilizing Lithium Metal Anodes
2023-08-11

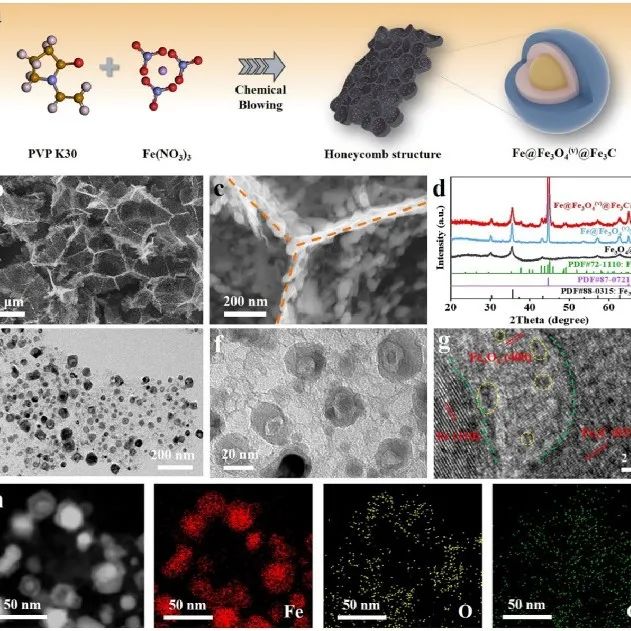
Ocean University of China Liu Wei AM: Utilizing the Coupling Effect of Oxygen Vacancies and Fe-C Bonds to Suppress Inactive Phase Transition for Long Cycle Life of Iron-based Anodes
2023-08-11
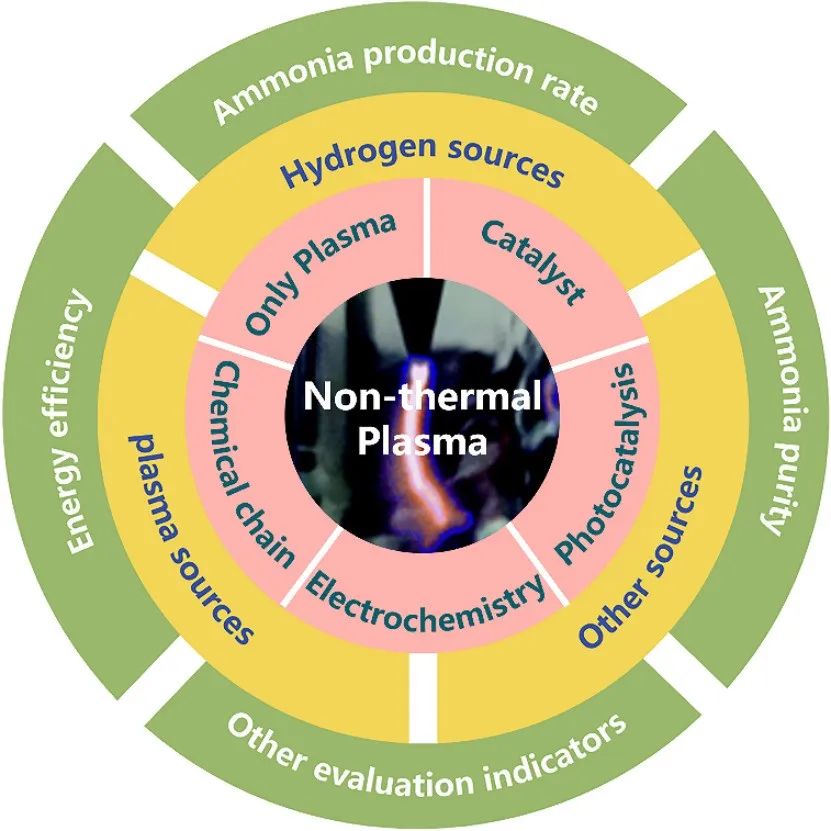
Non-Thermal Plasma-Assisted Ammonia Production
2023-08-11
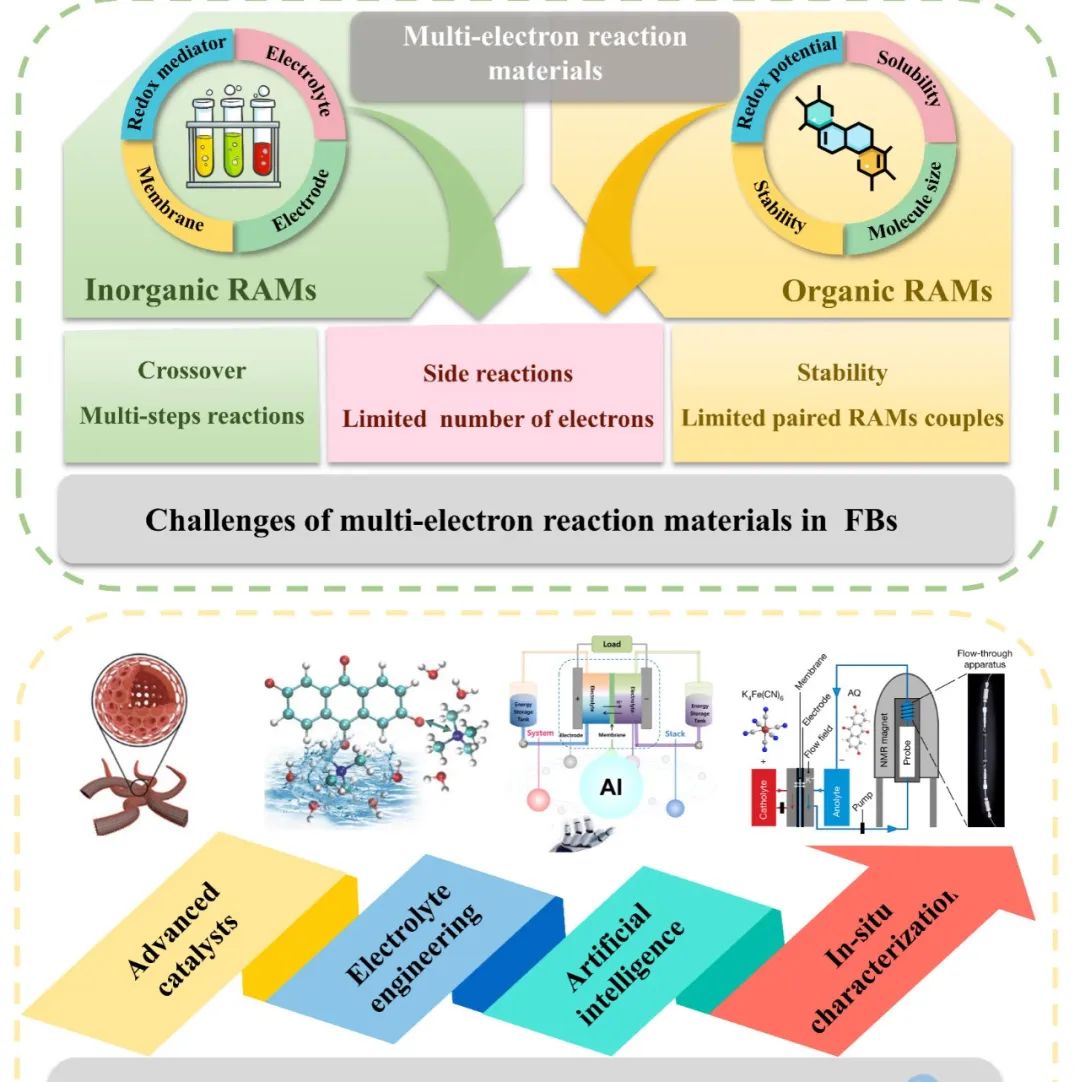
Dalian Institute of Chemical Physics’s Researcher Li Xianfeng & Zhang Changkun Next Energy: Multi-Electron Transfer Electrode Materials for High Energy Density Flow Batteries
2023-08-11
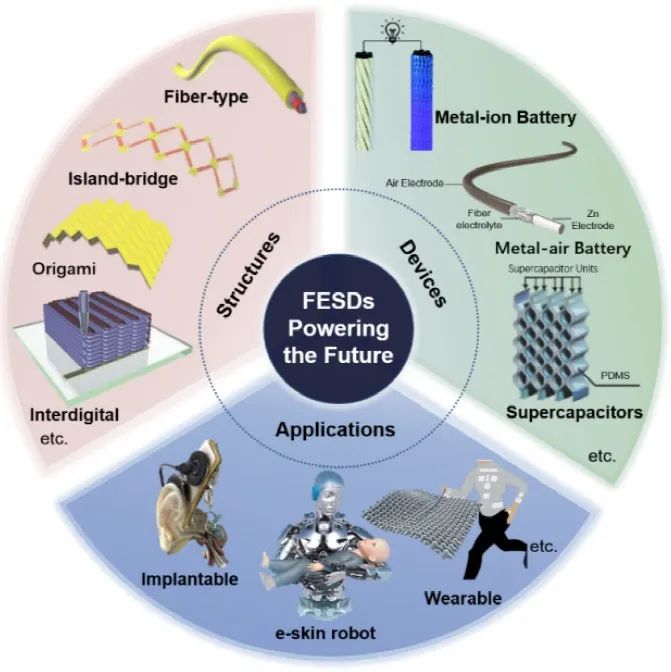
Northwestern Polytechnical University Guancao/Xu Qian’s latest Advanced Materials Review: Research Progress of Flexible Energy Storage Devices Driving the Future of Flexible Electronics
2023-08-11
Is Large-scale Application of Sodium-ion Batteries Just Around the Corner or Still a Long Way to Go?
2023-08-10

Liu Yongchang ACS Energy Lett.: New Sodium-ion Battery Cathode Na3.5Fe0.5VCr0.5(PO4)3 with Multi-Electron Reaction and Low Volume Strain
2023-08-10
