Myocardial ischemia-reperfusion injury (IRI) is a complex pathophysiological process that underlies persistent cardiac damage during cardiac surgery, heart transplantation, myocardial infarction, and cardiac arrest. During myocardial ischemia, the reduction in oxygen supply leads to a shift from fatty acid metabolism to anaerobic glycolysis, resulting in lactic acidosis that significantly decreases extracellular and intracellular pH to 6.0-6.5. In patients with acute myocardial infarction, the severity of metabolic acidosis is closely related to mortality, and acidosis leads to the activation of various transmembrane ion channels and receptors, among which ASIC1a, a variant of the ACCN1 gene, is the most pH-sensitive acid-sensing ion channel. Previous studies have shown that ASIC1a plays a key role in mediating ischemia-induced neuronal death, but its role in myocardial ischemia-reperfusion injury remains unclear.
Recently, a team from the University of Queensland and the University of New South Wales, including Glenn F. King, Peter S. Macdonald, and Nathan J.Palpant, published a research paper titled “Therapeutic Inhibition of Acid Sensing Ion Channel 1a Recovers Heart Function After Ischemia-Reperfusion Injury” in Circulation. This study provided compelling evidence that ASIC1a could be a target for myocardial ischemia-reperfusion protection, using human induced pluripotent stem cell-derived cardiomyocytes (hiPSC-CM) and in vitro and in vivo models of ischemia-reperfusion injury, indicating the potential application value of ASIC1a inhibitors in treating cardiac ischemic injury.

Figure 1: Article Cover
To determine whether ASIC1a plays a functional role during cardiac ischemia, the team first constructed ASIC1a knockout (KO) mice and then used the Langendorff method to assess the tolerance of KO and wild-type (WT) mouse hearts to IRI. They found that by the end of reperfusion, the LVDP and EDP of the ASIC1a-KO group were significantly higher than those of the WT group, while the ASIC1a-KO hearts also improved coronary blood flow recovery and reduced LDH production (Figure 2). These results suggest that ASIC1a is a key factor in myocardial IRI. Quantitative liquid chromatography-tandem mass spectrometry (LC-MS/MS) and GO analysis revealed that proteins related to redox and fatty acid metabolism were significantly increased in ASIC1a-KO compared to WT (Figure 2).
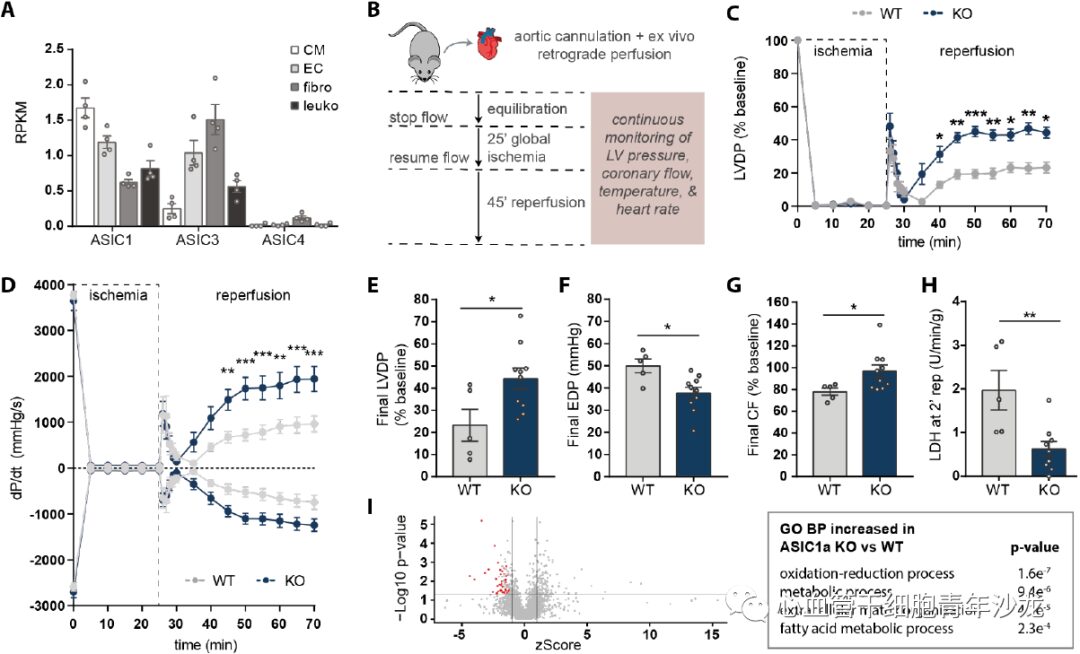
Figure 2: ASIC1a Gene Knockout Mitigates Isolated Mouse Heart Ischemia-Reperfusion Injury.
The team selected two venom-derived peptides, Hi1a and PcTx1, which are both effective and selective inhibitors of ASIC1a with no activity on other ASIC subtypes, and utilized analogs of PcTx1 (with mutations in two pharmacophore residues R27A/V32A), employing the Langendorff method to evaluate whether these ASIC1a inhibitors have protective effects against myocardial IRI. Compared to the control group, the Hi1a and PcTx1 groups showed increased LVDP and dP/dt, and at the end of reperfusion, both groups significantly improved LVDP and reduced EDP, while there was no significant difference between the PcTx1-R27A/V32A group and the control group (Figure 3). These data indicate that ASIC1a inhibitors can mitigate myocardial ischemia-reperfusion injury.
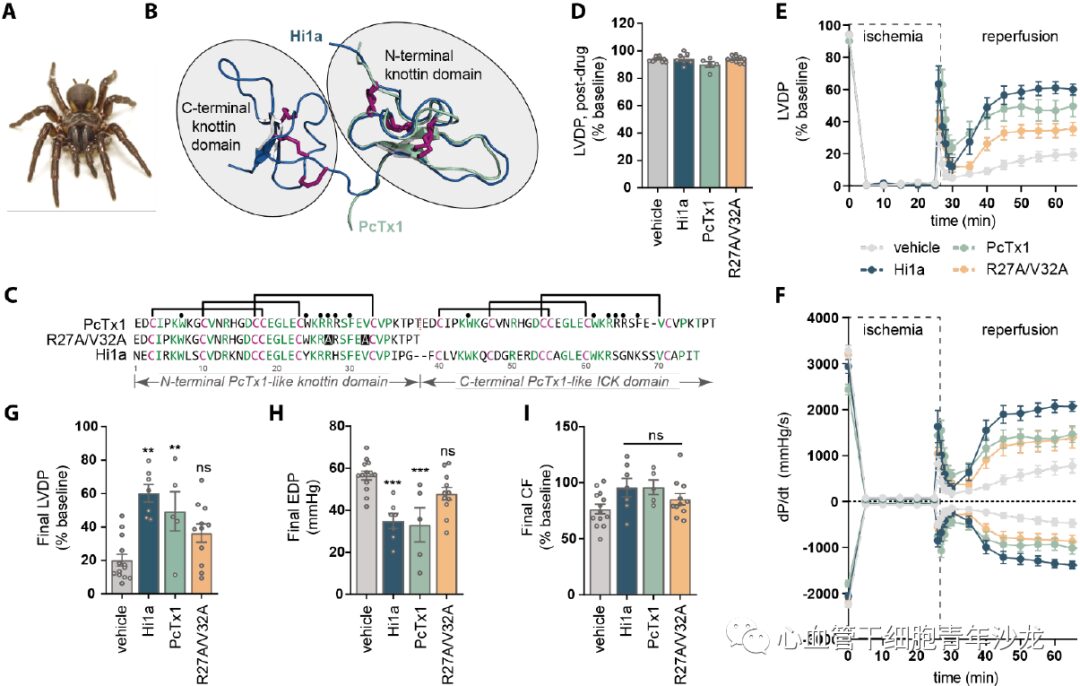
Figure 3: ASIC1a Inhibitors Mitigate Isolated Mouse Heart Ischemia-Reperfusion Injury.
The team supplemented the standard clinical cardiac preservation solution (Celsior) with 10 nM Hi1a during the 8-hour cold storage of isolated rat hearts. Compared to the control group using only Celsior, the hearts supplemented with Hi1a significantly improved aortic blood flow and cardiac output recovery (Figure 4), indicating that the ASIC1a inhibitor Hi1a has protective effects against IRI in a clinical model of long-term cold storage of donor hearts.
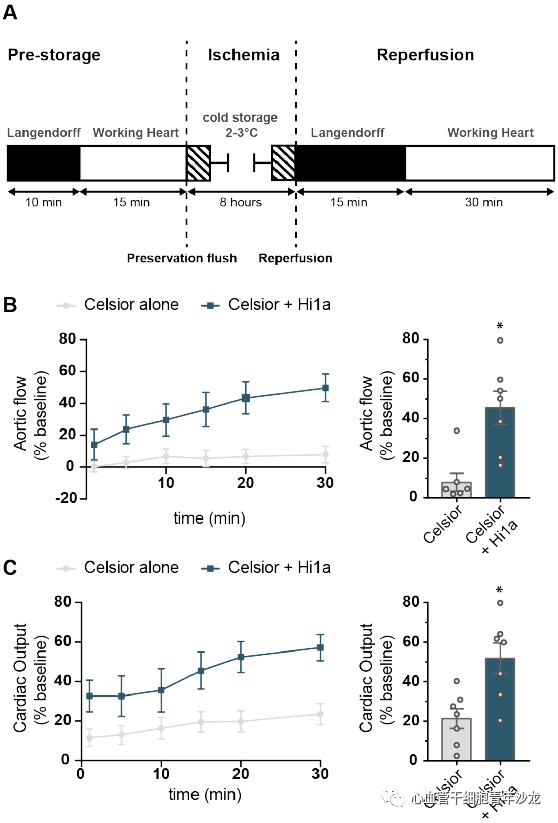
Figure 4: ASIC1a Inhibitor Hi1a Improves Cardiac Function Recovery After Long-Term Cold Storage of Isolated Rat Hearts
The team also evaluated the effect of Hi1a in a donation after circulatory death (DCD) animal model. Hi1a (10 nM) was tested as a single supplement combined with two clinically used supplements, glyceryl trinitrate (GTN) and erythropoietin (EPO), as positive controls, and the combination of Zon with GTN and EPO was also evaluated. Compared to the control group using only Celsior, the supplementation with Hi1a, Hi1a+GTN/EPO, or GTN/EPO/Zon significantly reduced LDH levels 30 minutes later, and parameters such as AF, CO, and PP were significantly improved, while the phosphorylation level of the key regulatory factor RIP3 related to necroptotic apoptosis pathways associated with IRI was reduced (Figure 5). These findings suggest that the targeted therapy of ASIC1a inhibitor Hi1a during clinical donor heart preservation has great clinical translational potential.
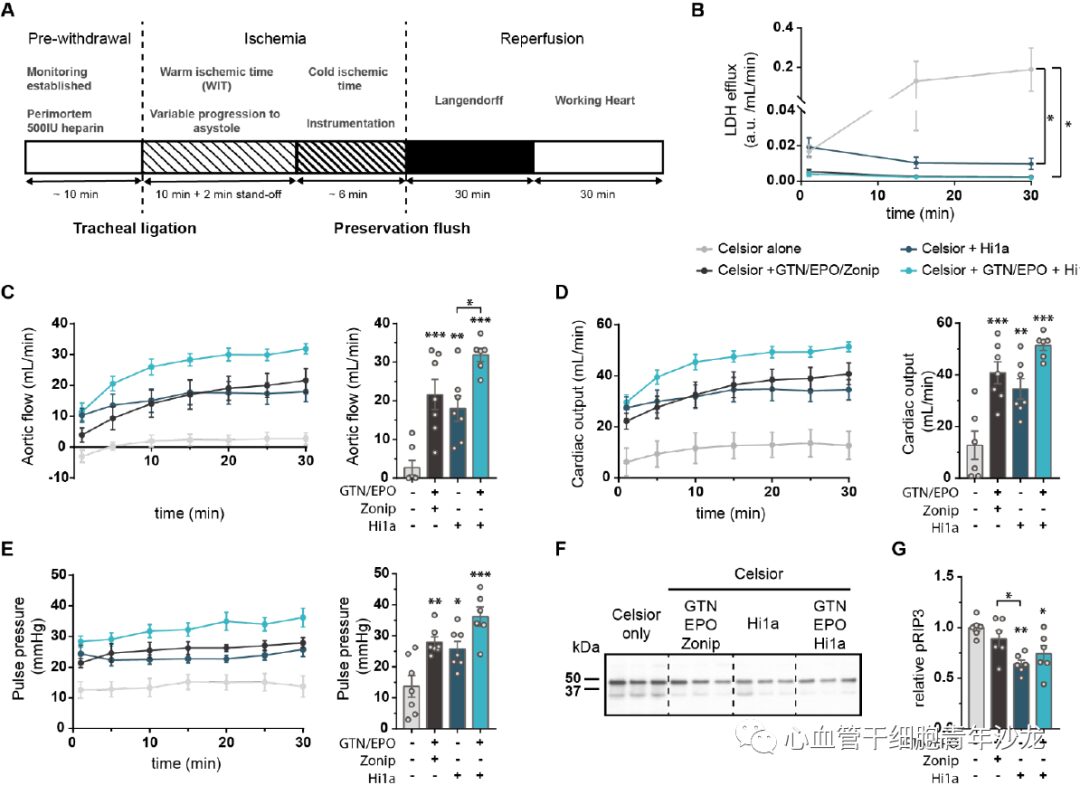
Figure 5: Hi1a Protects Rat Hearts in Circulatory Death Donation Model
The team also assessed the effects of Hi1a treatment in a mouse myocardial IRI model, finding that after Hi1a treatment, the fibrosis area of the mouse heart decreased, LVESVI and LVEDVI significantly increased, and EF improved significantly at 1 week. Although not significant at 4 weeks, the improvement was still maintained (p = 0.08), and there was no significant difference in CI compared to the sham surgery (Figure 6), indicating that ASIC1a inhibitors can mitigate cardiac remodeling after myocardial IRI.
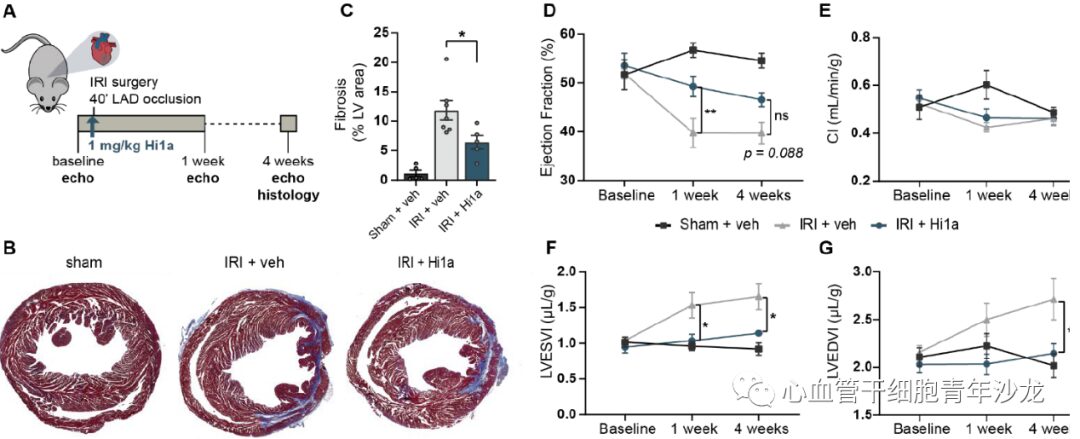
Figure 6: Hi1a Improves Post-Ischemic Cardiac Remodeling in Vivo
To assess the conservation of human ASIC1a biology, the team analyzed mRNA-seq and ribo-seq data collected from human left ventricular cardiac tissue, finding elevated expression levels of ASIC1 and ASIC3 in the human left ventricle, with ASIC1 expression levels increased in heart failure patients (Figure 7). The team also utilized genome-wide association studies (GWAS) to reveal significant associations between genetic variations at the ACCN1 locus and coronary artery disease, myocardial infarction, and small vessel ischemic stroke (Figure 7).
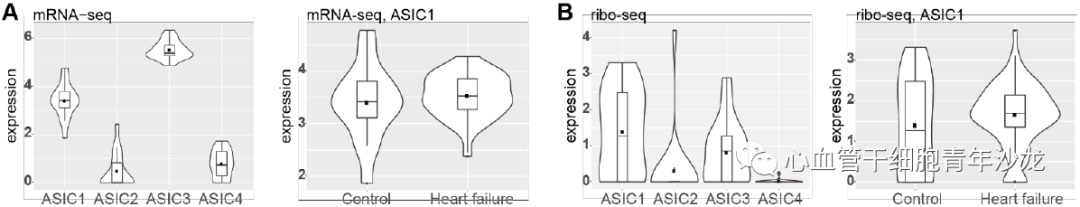
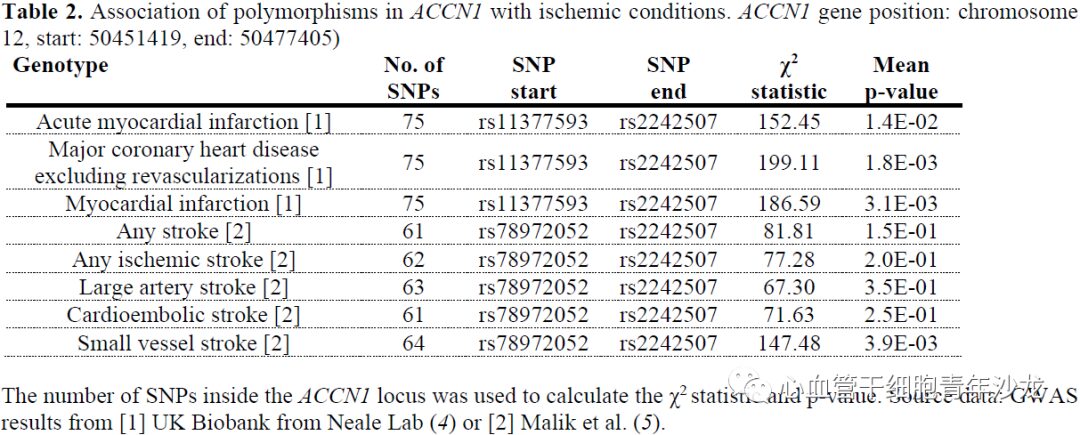
Figure 7: ACCN1 Gene Locus Polymorphism Associated with Ischemic Diseases
In vitro experiments using human induced pluripotent stem cell-derived cardiomyocytes (hiPSC-CM) revealed that ASIC1 is the most highly expressed ASIC subtype, with expression levels comparable to other cardiac ion channels. Additionally, neither Hi1a nor PcTx1 altered calcium amplitude or spontaneous beating rate. Simulating extracellular acidosis in vitro demonstrated that treatment with Hi1a or PcTx1 provided excellent cardiac protection, even at concentrations as low as 1 nM; similarly, treatment with 20 nM MitTx (a potent ASIC1a agonist) increased cell death under pH 7.4 and pH 6.0. Constructing an ischemia/acidosis-reperfusion in vitro model revealed that 10 nM Hi1a or PcTx significantly reduced cell death, indicating that ASIC1a inhibitors provide significant protective effects against cellular stress such as ischemic acidosis in vitro.
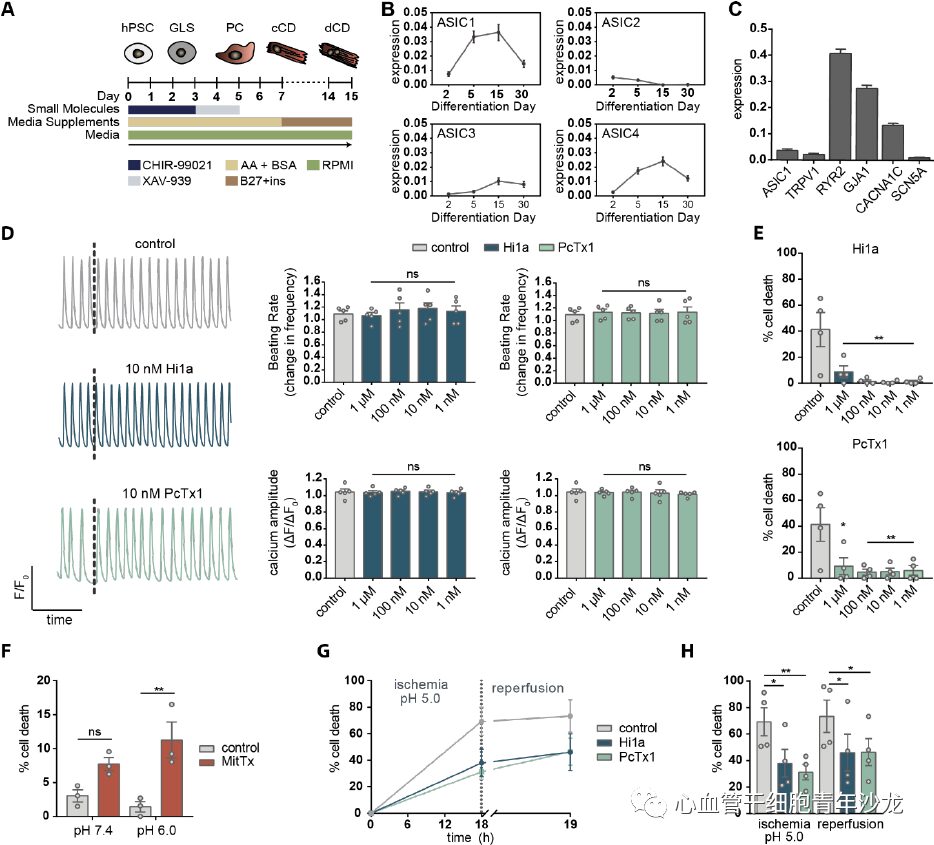
Figure 8: Hi1a Protects hiPSC-CM from Ischemic Injury
In conclusion, the authors demonstrated that ASIC1a is a key molecule in cell death during cardiac ischemia, and that ASIC1a inhibitors provide effective cardiac protection in rodent and human heart IRI models, offering new insights for the clinical treatment of ischemia-reperfusion injury related to ASIC1a inhibitors.
References:Redd Meredith A., Scheuer Sarah E., Saez Natalie J., Yoshikawa Yusuke., Chiu Han Sheng., Gao Ling., Hicks Mark., Villanueva Jeanette E., Joshi Yashutosh., Chow Chun Yuen., Cuellar-Partida Gabriel., Peart Jason N., See Hoe Louise E., Chen Xiaoli., Sun Yuliangzi., Suen Jacky Y., Hatch Robert J., Rollo Ben., Alzubaidi Mubarak A H., Maljevic Snezana., Quaife-Ryan Gregory A., Hudson James E., Porrello Enzo R., White Melanie Y., Cordwell Stuart J., Fraser John F., Petrou Steven., Reichelt Melissa E., Thomas Walter G., King Glenn F., Macdonald Peter S., Palpant Nathan J. (2021). Therapeutic Inhibition of Acid Sensing Ion Channel 1a Recovers Heart Function After Ischemia-Reperfusion Injury. Circulation. doi:10.1161/CIRCULATIONAHA.121.054360. PMID:34264749.








