

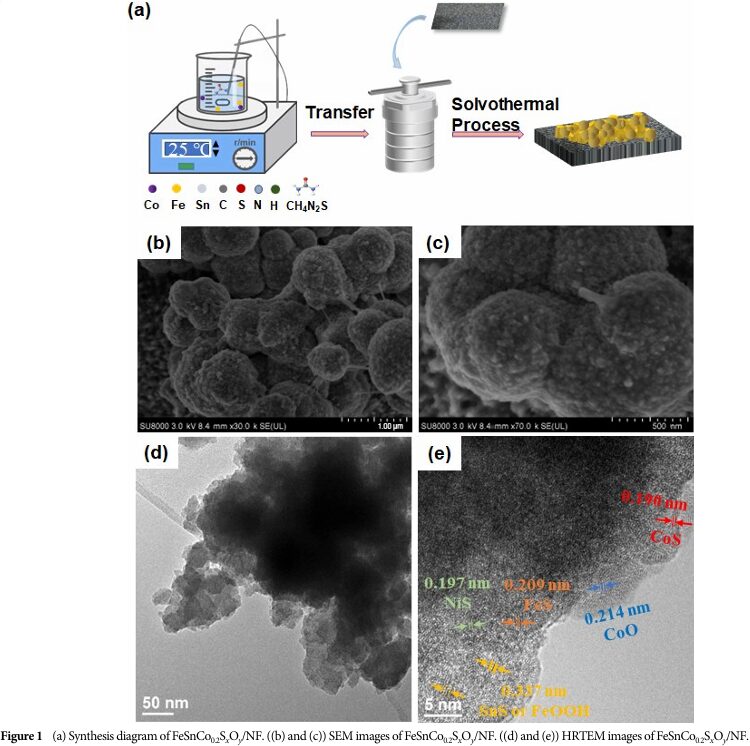
The researchers used nickel foam as a self-supporting substrate and synthesized a ternary metal sulfide/oxide heterostructure electrocatalyst (FeSnCo 0.2S x O y /NF) through a one-step solvothermal method.
This catalyst features a unique sulfide/oxide heterostructure interface that enhances catalytic performance.
02 Catalyst Performance Evaluation
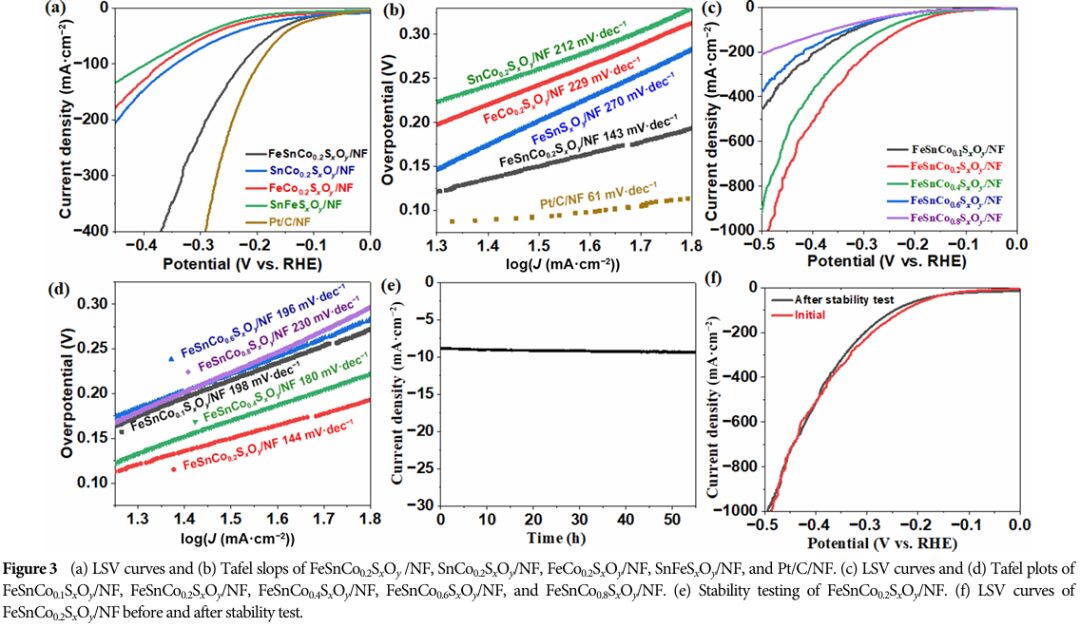
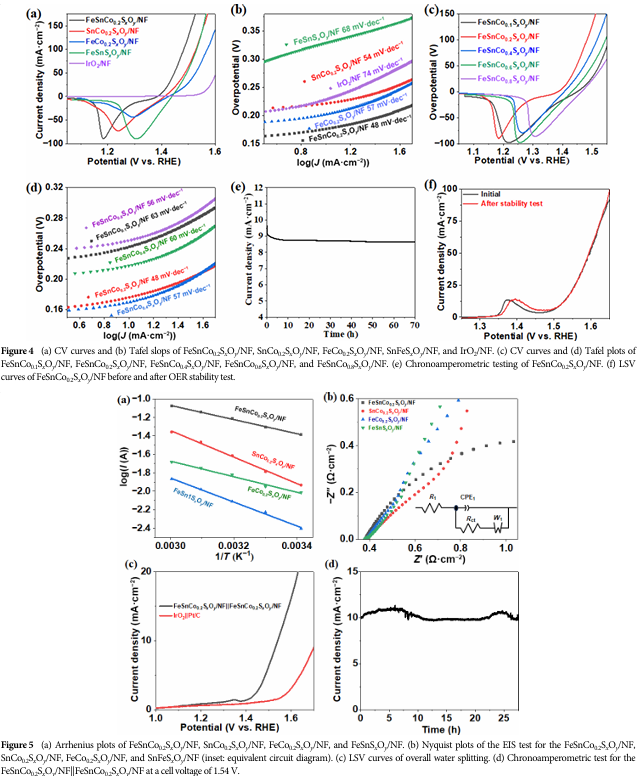
The catalyst exhibits excellent performance in both the oxygen evolution reaction (OER) and hydrogen evolution reaction (HER).
Especially in OER, it shows low overpotential and Tafel slope, indicating good catalytic activity and kinetic properties.
03 Role of Interface Engineering
The interface engineering strategy optimizes the catalytic performance of the catalyst by modulating the electronic structure at the heterostructure interface.
Moreover, the synergistic effect between sulfides and oxides further enhances the overall performance of the catalyst.
04 Catalyst Stability
The catalyst demonstrated good durability during long-term stability tests, with performance remaining largely unchanged after extended reactions, ensuring its long-term stable operation in practical applications.

05 Catalyst Morphology and Structure
Observations through scanning electron microscopy (SEM) and transmission electron microscopy (TEM) reveal that the catalyst exhibits unique morphological and structural features, which are closely related to its excellent catalytic performance.
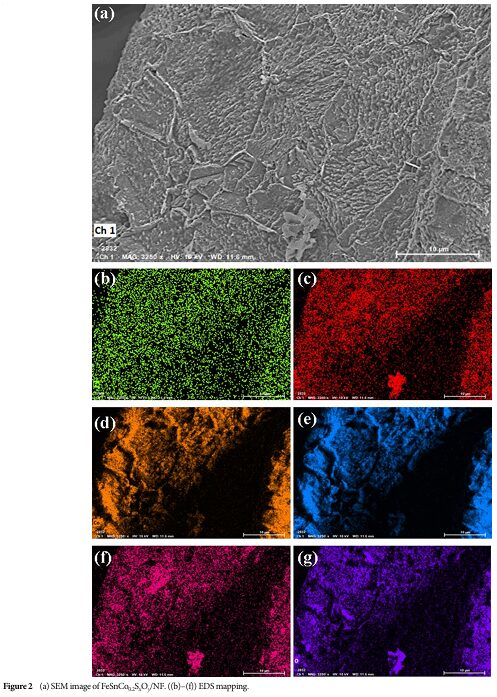
06 Catalyst Composition and Element Distribution
Energy dispersive spectroscopy (EDS) analysis indicates that various elements in the catalyst are uniformly distributed, aiding in efficient catalytic reactions.
07 Catalyst Catalytic Mechanism
The researchers explored the catalytic mechanism of the catalyst, attributing its excellent performance to abundant active sites and optimized reaction kinetics.
08 Illustrated Guide
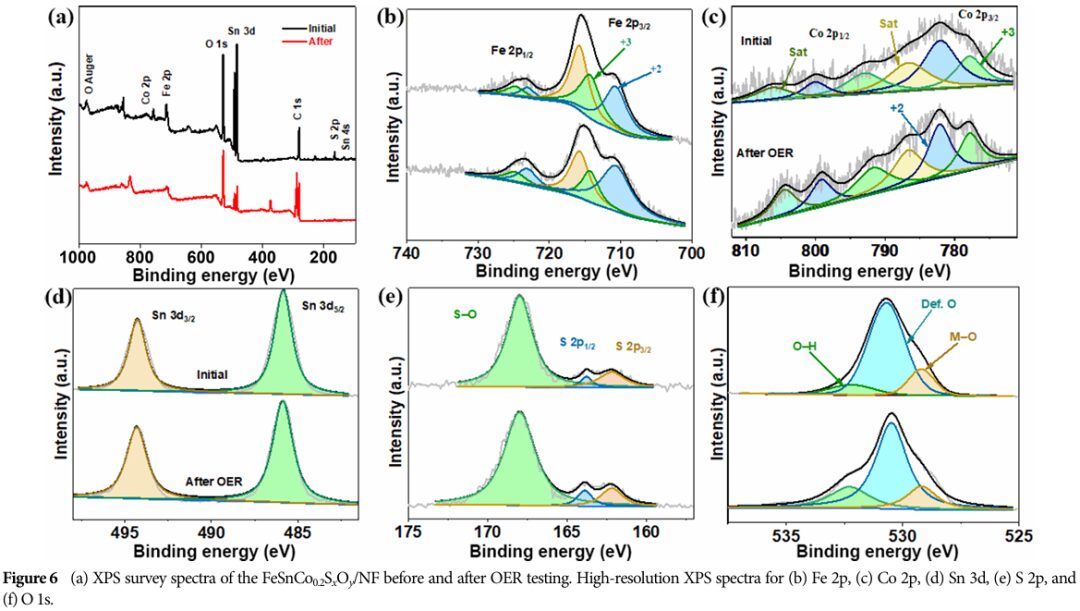

09 Conclusion
This article successfully designs and synthesizes an efficient non-precious metal bifunctional electrocatalyst through interface engineering strategies.
The catalyst exhibits excellent performance in both the oxygen evolution reaction and hydrogen evolution reaction, along with good stability and durability.
These research findings provide valuable references and insights for developing new high-efficiency water-splitting electrocatalysts.