iNature
Intense noise can damage the spiral ganglion neurons (SGN) in the inner ear, affecting axonal regeneration and leading to noise-induced hearing loss (NIHL).
On November 19, 2024, researchers from Fudan University, Bu Wenbo, Ren Dongdong, and Liu Yanyan published a paper titled“Ultrasound-Triggered NO Release to Promote Axonal Regeneration for Noise-Induced Hearing Loss Therapy” in ACS Nano.The study designed an ultrasound-triggered nitric oxide (NO) release to enhance the sprouting and regeneration of injured axons in SGN.
The authors utilized hollow silica nanoparticles loaded with nitrosated N-acetylcysteine to obtain HMSN-SNO. By using low-intensity ultrasound stimulation with bone-penetrating capabilities, they achieved controlled release of NO from HMSN-SNO in the cochlea. In mice with NIHL, there was a widespread loss of synaptic connections between hair cells and SGN observed within 24 hours after exposure to excessive noise. However, this loss could be reversed through combined therapy, restoring hearing function from 83.57 dB to 65.00 dB SPL. The multifunctional effect of HMSN-SNO eliminates reactive oxygen species (ROS) to reverse the pathological microenvironment while upregulating the CREB/BDNF/EGR1 signaling pathway, enhancing neural plasticity and promoting axonal regeneration of neurons. This finding emphasizes the potential of nanomedicine in regulating neural plasticity, promising to advance basic research and in-depth treatment of neurological diseases.
Noise-induced hearing loss (NIHL) is caused by prolonged or intense noise exposure, damaging the auditory system and is the leading cause of acquired hearing loss in industrial settings. In the inner ear, intense noise stimulation triggers an increase in mitochondrial aerobic respiration and the production of reactive oxygen species (ROS) in hair cells, leading to synaptic structural damage and axonal degeneration of spiral ganglion neurons (SGN). Although antioxidant therapies have shown clinical efficacy, the spontaneous regeneration of SGN axons and the establishment of synaptic connections with hair cells remain severely limited. Therefore, removing ROS after neuronal injury does not effectively restore hearing, and promoting axonal regeneration in SGN has become a new therapeutic target.
Currently, interventions for spiral neurons include cochlear implants or the application of neurotrophic factors such as BDNF to enhance the survival rate of SGN axons. Progress has been made, but their invasiveness or disruption of endogenous neurotrophic homeostasis fails to meet clinical needs. Nitric oxide (NO) serves as an intercellular messenger in vertebrates, participating in various physiological processes. Studies show that NO can rapidly diffuse into different tissues and physical barriers, acting as a promoter of nerve growth and regeneration. After intense noise stimulation, the expression of nNOS in the auditory epithelium decreases, leading to reduced endogenous NO synthesis. Additionally, oxidative stress-induced damage to the auditory epithelium results in decreased NOS1 expression. The findings indicate that endogenous NO levels are insufficient to stimulate axonal regeneration during noise injury, but NO is significant in repairing auditory damage.
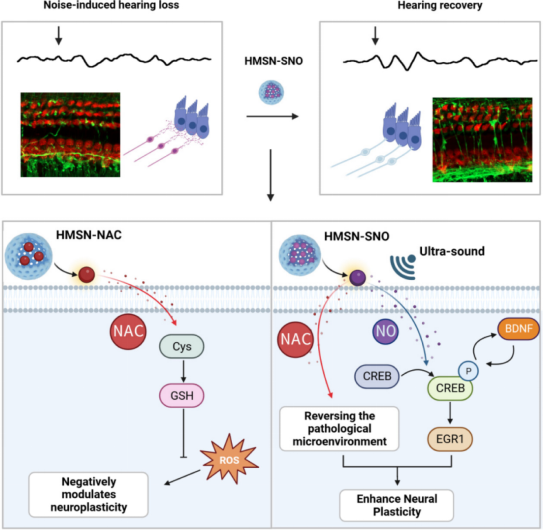
Figure 1: Schematic diagram of ultrasound-triggered NIHL promoting nerve regeneration (from ACS Nano)
The study proposes an ultrasound-triggered NO release strategy for the inner ear to promote axonal regeneration in SGN and achieve hearing restoration. N-acetylcysteine (NAC) is a powerful antioxidant that can effectively neutralize ROS and has excellent biocompatibility, approved for clinical prevention of hearing loss. NAC contains thiol groups that can form nitrosothiols through chemical modification, acting as NO donors. Hollow silica nanoparticles (HMSN) loaded with nitrosated N-acetylcysteine (NAC-SNO) can effectively protect the drug from external interference. The hollow silica nanoparticles can penetrate the inner ear through the round window membrane, thus the authors administered treatment via a single injection into the tympanic cavity. When stimulated by ultrasound from outside the skull, the low-energy sulfur-nitrogen bonds in NAC-SNO break, simultaneously releasing NAC and NO. In mice affected by NIHL, the authors observed that after 180 seconds of ultrasound stimulation with HMSN-SNO, the average hearing threshold in the frequency range of 8 to 24 kHz recovered from 83.57 to 65.00 dB SPL. Histological analysis of the inner ear showed enhanced regeneration of nerve fiber projections. Meanwhile, in vitro experiments indicated that the combination therapy significantly reduced apoptosis in C17.2 cells (a neural stem cell line), upregulating BDNF through CREB phosphorylation, subsequently activating EGR1 to promote neural plasticity. This method, utilizing low-intensity ultrasound and controllable release of compounds, shows therapeutic potential for acquired hearing loss caused by oxidative stress-related damage and a range of other neurological diseases.
References:
https://pubs.acs.org/doi/10.1021/acsnano.4c12676

—END—
The content is original from 【iNature】,
For reprinting, please indicate the source as 【iNature】
Add WeChat Group
iNature gathers 40,000 life science researchers and doctors. We have formed 80 comprehensive groups (16 PI groups and 64 doctoral groups), and also specialized groups in relevant fields (plants, immunology, cells, microbiology, gene editing, neurology, chemistry, physics, cardiovascular, oncology, etc.).Friendly Reminder: Please note your details when joining the group (format: school + major + name; if you are a PI/professor, please indicate as PI/Professor, otherwise it will be assumed you are a doctoral student). You can first add the editor’s WeChat ID (love_iNature), or long press the QR code to add the editor, and then join the relevant group, serious inquiries only.


Submission, collaboration, and reprint authorization inquiries
Please contact WeChat ID:13701829856 or email:[email protected]
If you find this article interesting, please click here!