Enzymatic MoS2 faces challenges such as unstable dispersion, low solubility, and biotoxicity in tissue repair.
On September 21, 2024, researchers from Sichuan University, Sun Yong and Han Xianglong, published a paper titled “Engineering Injectable Coassembled Hydrogel by Photothermal Driven Chitosan-Stabilized MoS2 Nanosheets for Infected Wound Healing” in ACS Nano.
The study designed an injectable self-healing hydrogel using chitosan and MoS2 through a stepwise co-assembly approach.
Polyphenol chitosan acted as a ‘structural stabilizer’ for MoS2 nanosheets, reconstructing well-dispersed MoS2@CSH nanosheets, improving the biocompatibility of traditional MoS2, enhancing its photothermal conversion and enzymatic activity, and ensuring efficient radical scavenging and antibacterial properties. Additionally, polyphenol chitosan served as a ‘molecular crosslinker’ by accelerating hydrogen bonding interactions between chitosan and multiple crosslinking reactions among polyphenols, forming an injectable near-infrared responsive MoS2@CSH hydrogel. The rapid self-healing ability of the MoS2@CSH hydrogel is beneficial for wound healing and dynamic adaptation. Results showed that the MoS2@CSH hydrogel significantly eliminated bacteria and accelerated angiogenesis in infected wounds. The photothermal driven co-assembly of MoS2 and polycations provides an alternative strategy for promoting infected wound healing.
 In recent years, ultrathin two-dimensional (2D) nanosheets of MoS2 have attracted widespread attention due to their excellent physical and chemical properties. Monolayer and few-layer MoS2 nanosheets are widely used in biomedicine due to their good biosafety, large specific surface area, excellent photothermal conversion efficiency, antibacterial properties, and enzymatic catalytic activity. However, due to their high surface energy, it is difficult to peel bulk MoS2 into ultrathin sheets, limiting their performance applications. Additionally, when MoS2 is exposed to oxygen, it oxidizes to form MoO42– and SO42–, losing its intrinsic properties in the process. Therefore, preventing the oxidation of peeled MoS2 nanosheets while fully peeling MoS2 to form functional monolayer or few-layer nanosheet structures is crucial for exerting its biological functions.
Currently, two main strategies are used to obtain MoS2 nanosheet structures. The first is to directly form monolayer nanosheets through chemical vapor deposition. After coating a suitable substrate surface with a molybdenum seed layer, sulfur-containing gases react with molybdenum to form monolayer or few-layer nanosheets. Although this method allows for the preparation of large-area, high-quality MoS2 nanosheets, it is costly. The second strategy involves directly peeling bulk MoS2 crystals into monolayer or few-layer nanosheets through solution exfoliation. However, ultrasonic dispersion in water or organic solvents requires the addition of exogenous polymers or surfactants to stabilize the exfoliated MoS2 nanosheets and prevent their re-stacking or aggregation. Due to the inefficient binding of dispersants to nanosheets under high concentration conditions, this method often requires biotoxic organic solvents, and secondary aggregation and stacking may occur, limiting the use of MoS2 in biomedical applications.Natural polyphenols exhibit a variety of reactive activities, capable of reacting with amino and thiol groups, or even chelating metal ions.
In recent years, ultrathin two-dimensional (2D) nanosheets of MoS2 have attracted widespread attention due to their excellent physical and chemical properties. Monolayer and few-layer MoS2 nanosheets are widely used in biomedicine due to their good biosafety, large specific surface area, excellent photothermal conversion efficiency, antibacterial properties, and enzymatic catalytic activity. However, due to their high surface energy, it is difficult to peel bulk MoS2 into ultrathin sheets, limiting their performance applications. Additionally, when MoS2 is exposed to oxygen, it oxidizes to form MoO42– and SO42–, losing its intrinsic properties in the process. Therefore, preventing the oxidation of peeled MoS2 nanosheets while fully peeling MoS2 to form functional monolayer or few-layer nanosheet structures is crucial for exerting its biological functions.
Currently, two main strategies are used to obtain MoS2 nanosheet structures. The first is to directly form monolayer nanosheets through chemical vapor deposition. After coating a suitable substrate surface with a molybdenum seed layer, sulfur-containing gases react with molybdenum to form monolayer or few-layer nanosheets. Although this method allows for the preparation of large-area, high-quality MoS2 nanosheets, it is costly. The second strategy involves directly peeling bulk MoS2 crystals into monolayer or few-layer nanosheets through solution exfoliation. However, ultrasonic dispersion in water or organic solvents requires the addition of exogenous polymers or surfactants to stabilize the exfoliated MoS2 nanosheets and prevent their re-stacking or aggregation. Due to the inefficient binding of dispersants to nanosheets under high concentration conditions, this method often requires biotoxic organic solvents, and secondary aggregation and stacking may occur, limiting the use of MoS2 in biomedical applications.Natural polyphenols exhibit a variety of reactive activities, capable of reacting with amino and thiol groups, or even chelating metal ions.
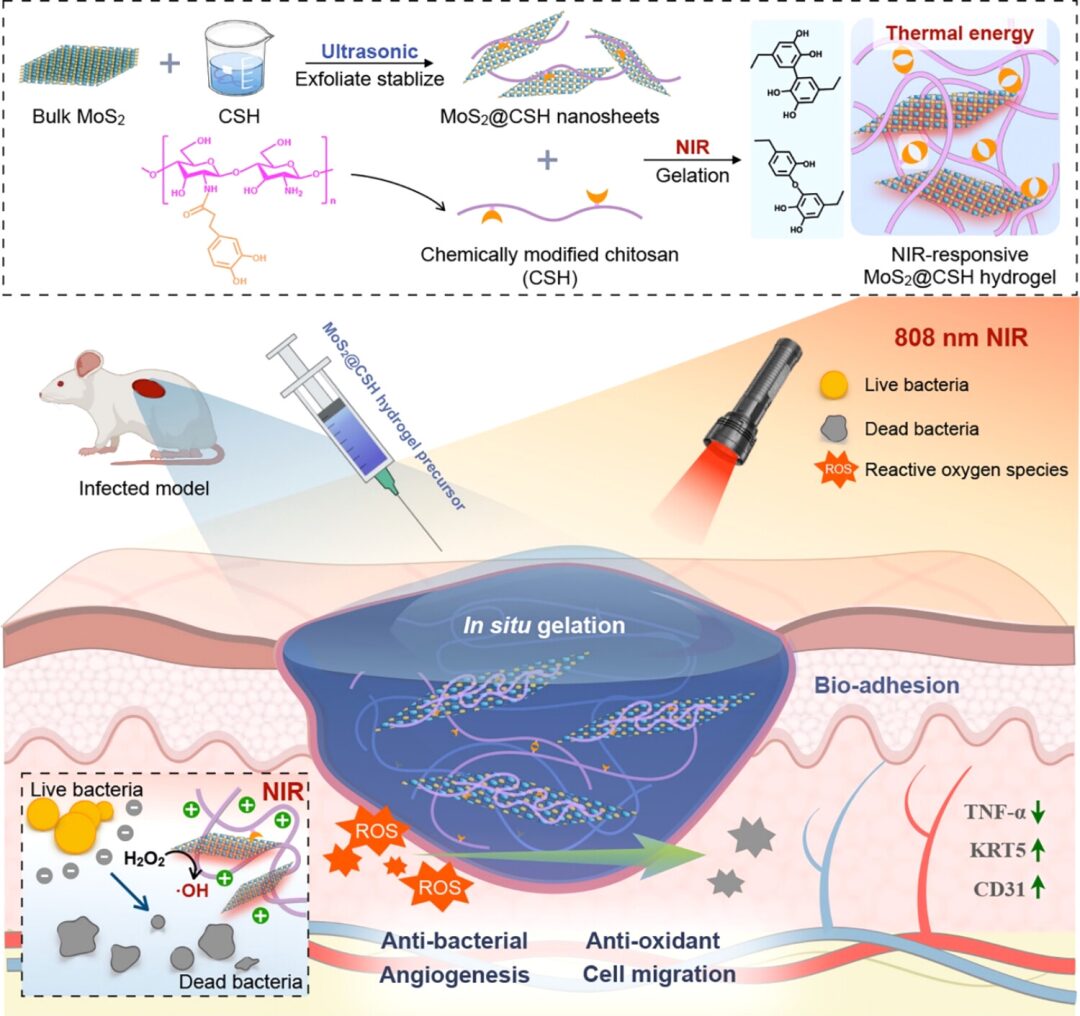 Figure 1: Schematic illustration of injectable MoS2@CSH hydrogel promoting infected wound healing (Excerpt from ACS Nano)
Polyphenolic substances (including catechin, epicatechin, and tannic acid) also demonstrate high binding energy when combined with MoS2. They bind to the MoS2 surface through their aromatic hydrophobic structures and hydrophobic interactions with MoS2, disrupting the van der Waals interactions between adjacent layers of MoS2, allowing the dispersed MoS2 nanosheets to be liquid-phase exfoliated. The phenolic hydroxyl groups combined with MoS2 can prevent the oxidation of MoS2 nanosheets in air and water. Meanwhile, the polyphenol-exfoliated MoS2 nanosheets also exhibit good enzymatic activity.
Under photothermal responsive conditions, the high heat and reactive oxygen species (ROS) generated damage bacterial cells by accelerating the oxidation/consumption of glutathione (GSH) within bacterial cells, thereby disrupting the bacterial defense system and the permeability and integrity of the bacterial membrane. However, MoS2 nanosheets tend to form aggregates in solution through hydrophobic interactions with each other. Due to the negatively charged surface sulfur atoms, the protonated amino groups of polycations chitosan can bind to them, maintaining the structural stability of MoS2 nanosheets through electrostatic interactions, while the abundant cations also synergistically enhance the antibacterial properties of MoS2 nanosheets. These findings confirm the important role of cationic polymers and polyphenols in maintaining the structural stability and enhancing the antibacterial function of exfoliated MoS2 nanosheets.
This study utilizes polyphenol chitosan as a ‘structural stabilizer’ for MoS2 nanosheets under ultrasonic dispersion and as a ‘molecular crosslinker’ under photothermal stimulation. Under ultrasonic dispersion conditions, polyphenol chitosan attaches to the surface of exfoliated MoS2 and co-assembles with it to form well-dispersed MoS2@CSH nanosheets. The assembled nanosheets are expected to achieve stronger solution stability and biocompatibility, as well as higher photothermal conversion and antibacterial efficiency. Furthermore, polyphenol chitosan stabilizes MoS2@CSH nanosheets by accelerating hydrogen bonding interactions between chitosan molecular chains and the multiple crosslinking reactions of polyphenols, forming an injectable near-infrared responsive MoS2@CSH hydrogel. The good wet adhesion and self-healing properties of the MoS2@CSH hydrogel enable it to adhere to damaged areas, providing long-term protection for wounds and creating a sufficient regenerative microenvironment for cell proliferation, angiogenesis, granulation tissue formation, and epithelial reformation. This injectable MoS2@CSH hydrogel with near-infrared (NIR) responsiveness has broad application prospects in the treatment of infected wounds.
https://pubs.acs.org/doi/10.1021/acsnano.4c08883
Figure 1: Schematic illustration of injectable MoS2@CSH hydrogel promoting infected wound healing (Excerpt from ACS Nano)
Polyphenolic substances (including catechin, epicatechin, and tannic acid) also demonstrate high binding energy when combined with MoS2. They bind to the MoS2 surface through their aromatic hydrophobic structures and hydrophobic interactions with MoS2, disrupting the van der Waals interactions between adjacent layers of MoS2, allowing the dispersed MoS2 nanosheets to be liquid-phase exfoliated. The phenolic hydroxyl groups combined with MoS2 can prevent the oxidation of MoS2 nanosheets in air and water. Meanwhile, the polyphenol-exfoliated MoS2 nanosheets also exhibit good enzymatic activity.
Under photothermal responsive conditions, the high heat and reactive oxygen species (ROS) generated damage bacterial cells by accelerating the oxidation/consumption of glutathione (GSH) within bacterial cells, thereby disrupting the bacterial defense system and the permeability and integrity of the bacterial membrane. However, MoS2 nanosheets tend to form aggregates in solution through hydrophobic interactions with each other. Due to the negatively charged surface sulfur atoms, the protonated amino groups of polycations chitosan can bind to them, maintaining the structural stability of MoS2 nanosheets through electrostatic interactions, while the abundant cations also synergistically enhance the antibacterial properties of MoS2 nanosheets. These findings confirm the important role of cationic polymers and polyphenols in maintaining the structural stability and enhancing the antibacterial function of exfoliated MoS2 nanosheets.
This study utilizes polyphenol chitosan as a ‘structural stabilizer’ for MoS2 nanosheets under ultrasonic dispersion and as a ‘molecular crosslinker’ under photothermal stimulation. Under ultrasonic dispersion conditions, polyphenol chitosan attaches to the surface of exfoliated MoS2 and co-assembles with it to form well-dispersed MoS2@CSH nanosheets. The assembled nanosheets are expected to achieve stronger solution stability and biocompatibility, as well as higher photothermal conversion and antibacterial efficiency. Furthermore, polyphenol chitosan stabilizes MoS2@CSH nanosheets by accelerating hydrogen bonding interactions between chitosan molecular chains and the multiple crosslinking reactions of polyphenols, forming an injectable near-infrared responsive MoS2@CSH hydrogel. The good wet adhesion and self-healing properties of the MoS2@CSH hydrogel enable it to adhere to damaged areas, providing long-term protection for wounds and creating a sufficient regenerative microenvironment for cell proliferation, angiogenesis, granulation tissue formation, and epithelial reformation. This injectable MoS2@CSH hydrogel with near-infrared (NIR) responsiveness has broad application prospects in the treatment of infected wounds.
https://pubs.acs.org/doi/10.1021/acsnano.4c08883

—END—
The content is original from [iNature],
Reprint must indicate the source from [iNature]
Add WeChat Group
iNature gathers 40,000 life science researchers and doctors. We have formed 80 comprehensive groups (16 PI groups and 64 doctoral groups), while also establishing specialized groups (plants, immunology, cells, microbiology, gene editing, neuroscience, chemistry, physics, cardiovascular, oncology, etc.).Kind reminder: Please note when joining the group (format: school + major + name; if you are a PI/professor, pleaseindicate that you are a PI/professor, otherwise it will be assumed you are a doctoral student, thank you).You can first add the editor’s WeChat ID (love_iNature), or long press the QR code to add the editor, and then join the relevant group. Serious inquiries only.


Submission, collaboration, and reprint authorization matters
Please contact WeChat ID:13701829856 or email:[email protected]
If you find this article interesting, please click here!




