
Photoacoustic Imaging (PAI) is an emerging technology that combines the high contrast of optical imaging with the deep penetration capabilities of ultrasound. It generates acoustic signals from tissues excited by laser, enabling functional and molecular-level imaging of living tissues. This technology does not require ionizing radiation and can assess blood oxygen levels through endogenous hemoglobin, as well as achieve targeted molecular imaging with exogenous contrast agents, showing great potential in fields such as tumor diagnosis and treatment, and cardiovascular diseases. This article focuses on a groundbreaking study: a research team developed a novel targeted contrast agent PAtrace and validated its precise imaging capabilities in an ovarian cancer model using a multispectral photoacoustic tomography (MSOT) system. Experiments showed that PAtrace not only separates from hemoglobin spectra to avoid interference with functional parameters but also enhances the accuracy of deep tissue imaging through surface fluence correction models.

A study published in Nature Communications has successfully developed a novel contrast agent PAtrace, which significantly enhances imaging performance by encapsulating indocyanine green (ICG) in liposomes to form J-aggregates. This technology was led by Cayla A. Wood, Sangheon Han, Konstantin V. Sokolov, and Richard R. Bouchard, and the research results were formally published under the title Clinically translatable quantitative molecular photoacoustic imaging with liposome-encapsulated ICG J-aggregates.
Key Findings
01Development and Validation of Targeted Contrast Agent PAtrace
The research team designed a nanoprobes PAtrace that targets folate receptor alpha (FRα), with its spectral absorption peak (750-800nm) completely separated from hemoglobin (400-600nm), avoiding signal interference from traditional contrast agents and endogenous substances. In vitro cell experiments showed that PAtrace achieved specific binding in FRα high-expressing ovarian cancer cells (such as A2780 cell line), with photoacoustic signal intensity 3.2 times that of the control group.
In in vivo experiments, the researchers constructed an ovarian cancer mouse model and performed multi-wavelength excitation (680-950nm) using the inVision 256-TF MSOT system. The results showed that the signal accumulation of PAtrace in the tumor region was significantly higher than in normal tissues, and it highly matched the tumor boundaries located by MRI. Spectral unmixing analysis further confirmed that PAtrace signals accounted for 65%, while hemoglobin signals only accounted for 35%, indicating its excellent molecular specificity.
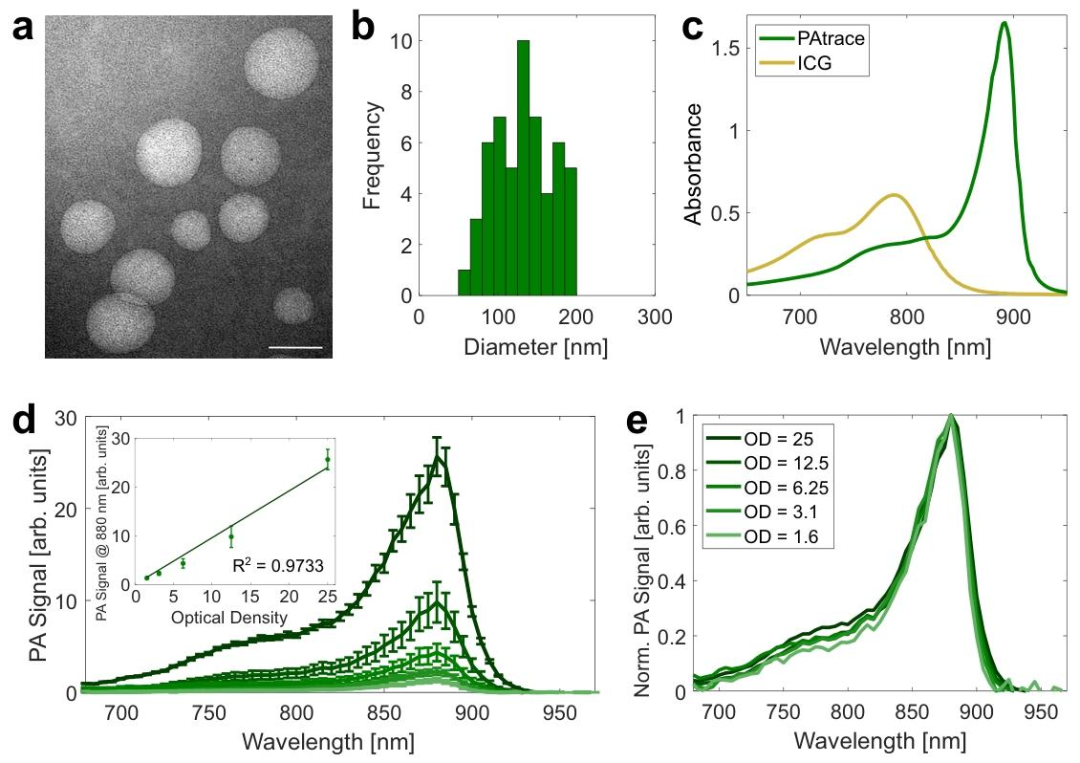
Figure 1 Analysis of PAtrace Characteristics
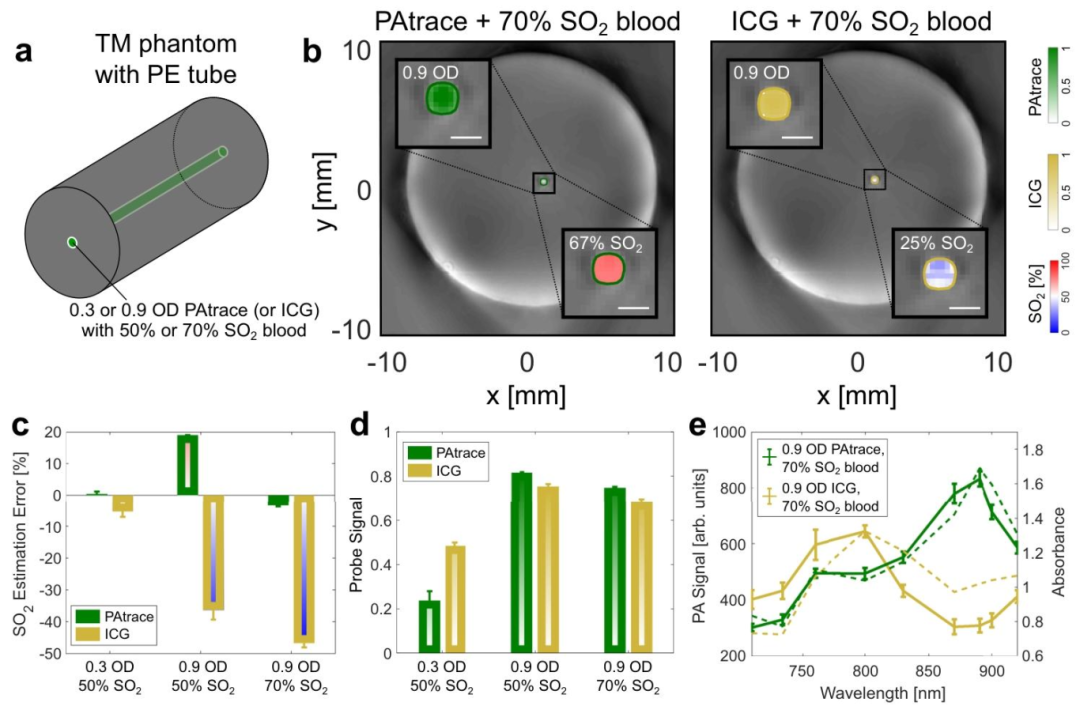
Figure 2 Comparison of PAtrace and ICG in Blood-Containing Phantoms
02
Simultaneous Functional and Molecular Imaging
The unique advantage of photoacoustic imaging lies in its ability to simultaneously obtain physiological function and molecular information. In the experiment, the research team calculated the blood oxygen saturation (SO2) in the tumor region through dual-wavelength (532nm and 558nm) excitation, finding that the SO2 value at the tumor center (32±5%) was significantly lower than at the edge (58±8%), reflecting the hypoxic characteristics of the tumor microenvironment. Meanwhile, the targeted signal of PAtrace was positively correlated with tumor vascular density (R²=0.89), indicating its capability to simultaneously assess tumor angiogenesis and molecular expression status.
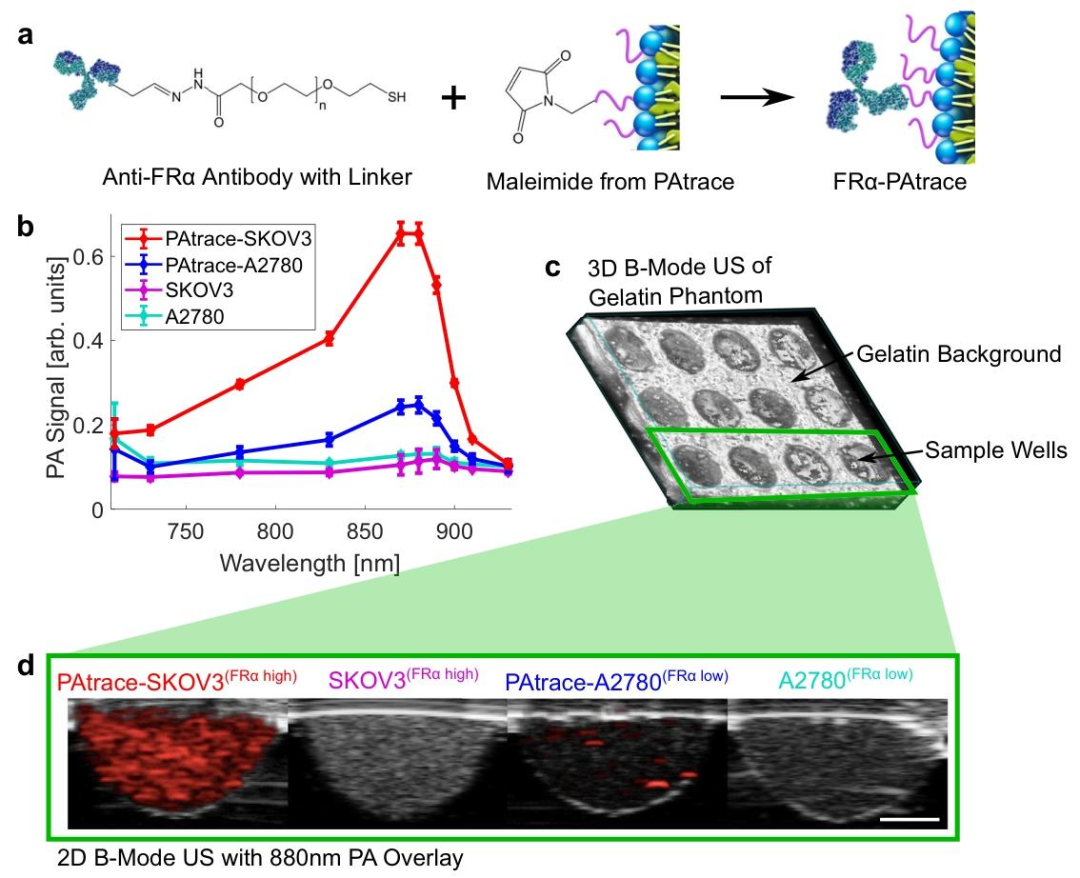
Figure 3 PA Signal from Naked Cells
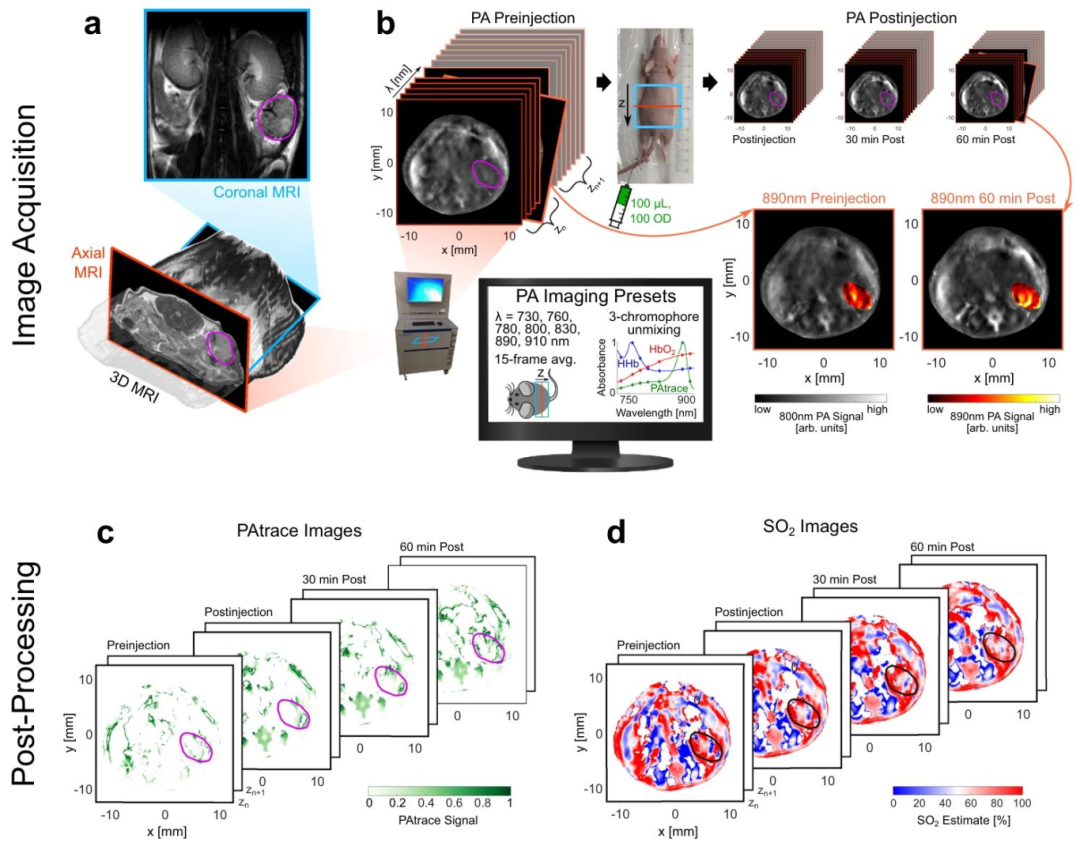
Figure 4 Summary of In Vivo PA Imaging Research Methods
03
Synergistic Effects of Multimodal Imaging
To enhance imaging accuracy, the research team combined photoacoustic imaging with B-mode ultrasound and MRI:
B-mode ultrasound-assisted localization: The tumor boundary was delineated by B-mode ultrasound, reducing the ROI error of photoacoustic signal analysis to ±0.1mm.
MRI fusion validation: The tumor volume measured by MRI (320±40mm³) was highly consistent with the photoacoustic reconstruction result (305±35mm³), validating the anatomical localization accuracy of photoacoustic imaging.
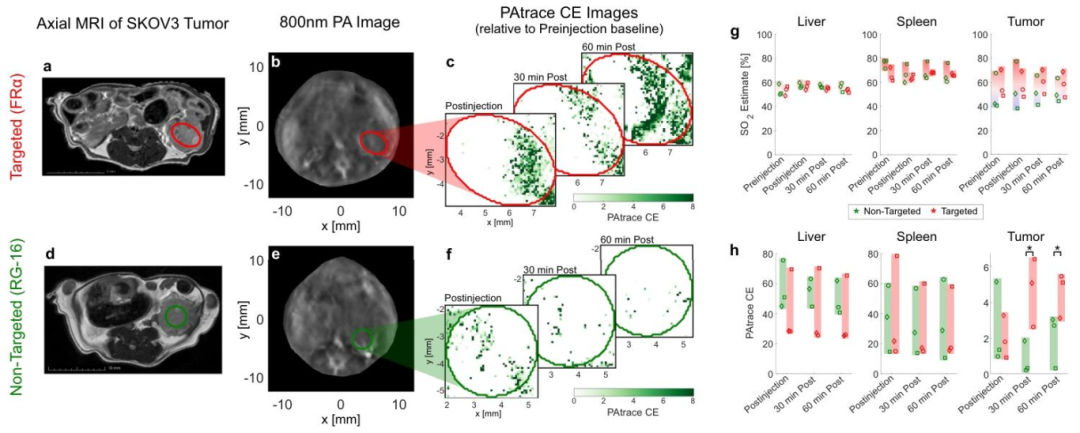
Figure 5 In Vivo PA Imaging Specificity of Targeted FRα-PAtrace in an In Situ Ovarian Cancer Model
Innovations and Highlights
01
Breakthrough in Spectral Separation Technology
In traditional photoacoustic imaging, exogenous contrast agents are prone to overlap with hemoglobin spectra, leading to misjudgment of functional parameters (such as SO2). This study optimized the absorption spectrum of PAtrace to completely separate it from hemoglobin and utilized the spectral unmixing algorithm of MSOT to achieve precise differentiation between contrast agent signals and physiological signals. This breakthrough provides a technical foundation for multimolecular imaging, such as simultaneously analyzing blood oxygen status and HER2 receptor expression in breast cancer detection.
02
Optimization of Deep Tissue Imaging
Light attenuation in tissues can lead to distortion of deep signals. The research team developed a surface fluence correction model to simulate the propagation path of light in tissues, compensating for signal attenuation caused by scattering and absorption. In a 10mm deep agarose gel model, the error of the corrected photoacoustic signal decreased from 28% to 5%, significantly improving quantitative accuracy. This technology makes photoacoustic imaging applicable in thick tissues such as the liver and breast.
03
Clinical Value of Non-Ionizing Radiation
Compared to ionizing radiation imaging technologies like PET and CT, photoacoustic imaging is safer, especially suitable for long-term monitoring and repeated examinations. For example, in evaluating ovarian cancer treatment, photoacoustic imaging can perform non-invasive scans every three days to track the normalization process of tumor blood vessels, while traditional MRI requires intervals of more than two weeks to reduce radiation risk.
Conclusion and Outlook
Photoacoustic imaging, with its non-invasive, high-resolution, and multimodal compatibility features, is reshaping the field of medical imaging. This study, through the development of the targeted contrast agent PAtrace and optimization of imaging algorithms, has overcome two major bottlenecks of traditional photoacoustic technology—spectral interference and deep signal attenuation—providing new tools for precise diagnosis and treatment of diseases like ovarian cancer. In the future, with the development of photoacoustic endoscopy and portable devices, this technology is expected to be popularized in primary healthcare, enabling early tumor screening and real-time monitoring of treatment responses.
However, the clinical translation of photoacoustic imaging still faces challenges: for instance, the biocompatibility of contrast agents needs further validation, and the imaging speed (currently about 1 frame per second) needs to be improved to meet dynamic monitoring needs. Additionally, the development of cross-modal data fusion algorithms (such as photoacoustic-CT/MRI combined analysis) will be key to enhancing diagnostic efficacy. With advancements in materials science and artificial intelligence, photoacoustic imaging is expected to become a core technology in the next generation of personalized medicine, realizing the vision of “non-invasive insight into internal lesions.”
Paper Information
Disclaimer: This article is for academic purposes only.
Wood CA, Han S, Kim CS, Wen Y, Sampaio DRT, Harris JT, Homan KA, Swain JL, Emelianov SY, Sood AK, Cook JR, Sokolov KV, Bouchard RR. Clinically translatable quantitative molecular photoacoustic imaging with liposome-encapsulated ICG J-aggregates. Nat Commun. 2021 Sep 13;12(1):5410.
DOI:10.1038/s41467-021-25452-3.


Welcome to add WeChat for more information~
Please note your name, organization, and other information when adding!

 Optical ImagingFollow us to learn more about optical knowledge!
Optical ImagingFollow us to learn more about optical knowledge!