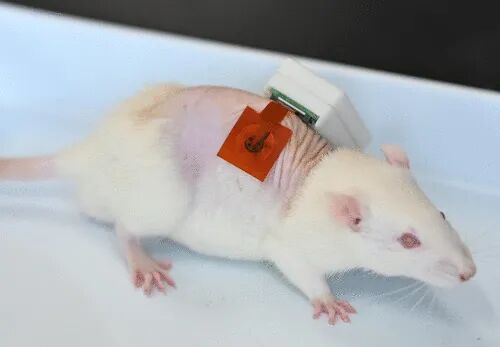
 Wearable wound exudate sensors hold great promise for dynamic measurements of valuable biomarkers. However, existing sensors fail to achieve fully integrated, skin-adhered, and dynamic detection of raw wound exudate oxygen (O2), which is closely related to wound conditions and is crucial for wound healing. Here, we report a fully integrated wearable biomimetic microfluidic wound tracker capable of skin-adhered biomimetic microfluidic sampling of wound exudate, dynamic monitoring of wound exudate O2, as well as uric acid, lactate, pH, and temperature, achieved through seamless integration of specially designed microfluidic, sensing, and electronic modules for wireless control. We tested the device in mouse models with both inoculated and non-inoculated bacterial wounds. We further evaluated its wound management potential during the wound healing process in infected diabetic mice through control experiments associated with local hyperbaric O2 therapy. This article comes from the research group of Zhou Ming at Northeast Normal University and was published today in ACS Nano, titled: A Fully Integrated Wearable Biomimetic Microfluidic Wound Tracker for In Situ Dynamic Monitoring of Wound Exudate Oxygen. Wound infection is a major challenge in global healthcare, affecting millions and leading to increased morbidity, limb loss, and even death. Early and accurate assessment of wound conditions is crucial for implementing personalized treatment interventions. However, current clinical practices for wound monitoring, diagnosis, and daily healing management heavily rely on visual inspections by healthcare professionals or wound culture tests. These traditional methods are often subjective, inaccurate, time-consuming, and fail to capture the dynamic physicochemical microenvironment of wounds, potentially leading to treatment delays or mismanagement. Epidermal wound exudate is a biological fluid that can be non-invasively collected from the skin surface, containing various biomarkers, including electrolytes, metabolites, cytokines, and growth factors. Changes in these biomarkers provide valuable insights into wound conditions, including healing stages and infection status. Among them, oxygen (O2) is a key biomarker that plays a critical role in essential physiological and cellular signaling processes necessary for wound healing. Notably, low O2 concentrations are closely associated with exacerbated inflammation, severity of infection, and impaired healing. Therefore, timely, precise, skin-adhered, and dynamic analysis of O2 in wound exudate represents a promising non-invasive approach for assessing wound conditions, facilitating accurate diagnosis, and effective treatment planning. A fully functional integrated wearable device that provides comprehensive functionality without external equipment is regarded as the ultimate goal of modern technological advancements. Developing a wearable device for monitoring wound exudate O2 concentration based on fully integrated design will enable skin adherence, wireless, non-invasive, and dynamic tracking of O2 concentration, significantly improving wound management. In 2015, a collaborative team of researchers from Tufts University, Harvard Medical School, MIT, Harvard University, Purdue University, and Abdulaziz University took the first step towards fully integrated wearable O2 concentration sensors. Their design was a flexible wireless bandage that demonstrated great potential for real-time wound oxygenation monitoring. However, this “proof-of-concept” device primarily focused on system integration and miniaturization without utilizing raw wound exudate for in vivo or in vitro testing. Therefore, its suitability for in vivo testing remains uncertain. Subsequently, Koh and collaborators from Binghamton University and United Health Services Hospital developed a wearable O2 sensor for analyzing raw wound exudate, known as the electronic extracellular matrix platform. However, this sensor has some limitations: its performance has not been validated against the “gold standard” methods; no human testing has been conducted; and the design has not been fully integrated. These shortcomings prevent it from meeting the requirements for autonomous, skin-adhered, and real-time tracking of O2 levels in wound exudate. Overall, these challenges highlight the urgent need to design and construct a truly integrated wearable wound exudate O2 sensor capable of precise, in situ, and dynamic monitoring. Here, we introduce the first fully integrated wearable biomimetic microfluidic wound tracker (FIWBMWT) for non-invasive, skin-adhered, dynamic monitoring of wound exudate O2 and other key biomarkers, including uric acid (UA), lactate, pH, and temperature. This functionality is achieved through seamless integration of microfluidic, sensing, and electronic modules. The biomimetic microfluidic system is inspired by the glandular hairs of the tomato (Solanum lycopersicum), ensuring effective in situ transport and collection of wound exudate. A nano-functionalized multiplexed sensor array can detect the aforementioned biomarkers with high sensitivity and accuracy. The performance of FIWBMWT was evaluated through long-term observations in mouse models of inoculated and non-inoculated bacterial wounds. The captured biomarker data revealed changes in the wound microenvironment over time and contributed to understanding the severity of wounds, including detecting exacerbated wound infections associated with hyperglycemia in diabetic mice. FIWBMWT is capable of simultaneous and multiplexed analysis of wound exudate O2 and other biomarkers, making it an ideal tool for preventing wound infections, personalized diagnostics, and customized treatment interventions.
Wearable wound exudate sensors hold great promise for dynamic measurements of valuable biomarkers. However, existing sensors fail to achieve fully integrated, skin-adhered, and dynamic detection of raw wound exudate oxygen (O2), which is closely related to wound conditions and is crucial for wound healing. Here, we report a fully integrated wearable biomimetic microfluidic wound tracker capable of skin-adhered biomimetic microfluidic sampling of wound exudate, dynamic monitoring of wound exudate O2, as well as uric acid, lactate, pH, and temperature, achieved through seamless integration of specially designed microfluidic, sensing, and electronic modules for wireless control. We tested the device in mouse models with both inoculated and non-inoculated bacterial wounds. We further evaluated its wound management potential during the wound healing process in infected diabetic mice through control experiments associated with local hyperbaric O2 therapy. This article comes from the research group of Zhou Ming at Northeast Normal University and was published today in ACS Nano, titled: A Fully Integrated Wearable Biomimetic Microfluidic Wound Tracker for In Situ Dynamic Monitoring of Wound Exudate Oxygen. Wound infection is a major challenge in global healthcare, affecting millions and leading to increased morbidity, limb loss, and even death. Early and accurate assessment of wound conditions is crucial for implementing personalized treatment interventions. However, current clinical practices for wound monitoring, diagnosis, and daily healing management heavily rely on visual inspections by healthcare professionals or wound culture tests. These traditional methods are often subjective, inaccurate, time-consuming, and fail to capture the dynamic physicochemical microenvironment of wounds, potentially leading to treatment delays or mismanagement. Epidermal wound exudate is a biological fluid that can be non-invasively collected from the skin surface, containing various biomarkers, including electrolytes, metabolites, cytokines, and growth factors. Changes in these biomarkers provide valuable insights into wound conditions, including healing stages and infection status. Among them, oxygen (O2) is a key biomarker that plays a critical role in essential physiological and cellular signaling processes necessary for wound healing. Notably, low O2 concentrations are closely associated with exacerbated inflammation, severity of infection, and impaired healing. Therefore, timely, precise, skin-adhered, and dynamic analysis of O2 in wound exudate represents a promising non-invasive approach for assessing wound conditions, facilitating accurate diagnosis, and effective treatment planning. A fully functional integrated wearable device that provides comprehensive functionality without external equipment is regarded as the ultimate goal of modern technological advancements. Developing a wearable device for monitoring wound exudate O2 concentration based on fully integrated design will enable skin adherence, wireless, non-invasive, and dynamic tracking of O2 concentration, significantly improving wound management. In 2015, a collaborative team of researchers from Tufts University, Harvard Medical School, MIT, Harvard University, Purdue University, and Abdulaziz University took the first step towards fully integrated wearable O2 concentration sensors. Their design was a flexible wireless bandage that demonstrated great potential for real-time wound oxygenation monitoring. However, this “proof-of-concept” device primarily focused on system integration and miniaturization without utilizing raw wound exudate for in vivo or in vitro testing. Therefore, its suitability for in vivo testing remains uncertain. Subsequently, Koh and collaborators from Binghamton University and United Health Services Hospital developed a wearable O2 sensor for analyzing raw wound exudate, known as the electronic extracellular matrix platform. However, this sensor has some limitations: its performance has not been validated against the “gold standard” methods; no human testing has been conducted; and the design has not been fully integrated. These shortcomings prevent it from meeting the requirements for autonomous, skin-adhered, and real-time tracking of O2 levels in wound exudate. Overall, these challenges highlight the urgent need to design and construct a truly integrated wearable wound exudate O2 sensor capable of precise, in situ, and dynamic monitoring. Here, we introduce the first fully integrated wearable biomimetic microfluidic wound tracker (FIWBMWT) for non-invasive, skin-adhered, dynamic monitoring of wound exudate O2 and other key biomarkers, including uric acid (UA), lactate, pH, and temperature. This functionality is achieved through seamless integration of microfluidic, sensing, and electronic modules. The biomimetic microfluidic system is inspired by the glandular hairs of the tomato (Solanum lycopersicum), ensuring effective in situ transport and collection of wound exudate. A nano-functionalized multiplexed sensor array can detect the aforementioned biomarkers with high sensitivity and accuracy. The performance of FIWBMWT was evaluated through long-term observations in mouse models of inoculated and non-inoculated bacterial wounds. The captured biomarker data revealed changes in the wound microenvironment over time and contributed to understanding the severity of wounds, including detecting exacerbated wound infections associated with hyperglycemia in diabetic mice. FIWBMWT is capable of simultaneous and multiplexed analysis of wound exudate O2 and other biomarkers, making it an ideal tool for preventing wound infections, personalized diagnostics, and customized treatment interventions.
Design and Overview of the Fully Integrated Wearable Biomimetic Microfluidic Wound Tracker (FIWBMWT)
Figure 1A shows a schematic of the skin-adhered FIWBMWT, designed for wireless, close monitoring of O2 (O2) in wound exudate and other key biomarkers, including uric acid (UA), lactate, pH, and temperature. These biomarkers are closely related to an individual’s wound condition and provide critical information to assist healthcare professionals in assessing wound status and providing timely, personalized management strategies. FIWBMWT consists of three main components: a microfluidic module for in situ biomimetic collection and transport of raw wound exudate, a sensing module for skin conformal analysis of biomarkers, and an electronic module for device control and real-time wireless signal communication (Figure 1B-G). The biomimetic microfluidic module integrates an adhesive layer for body attachment and a wedge-shaped surface with superhydrophobic and superhydrophilic properties to facilitate effective collection and directional transport of wound exudate (Figure 1B-D and Figure S1-S5). The sensing module includes a polyimide (PI) layer for fixing sensors, a sensor array for analyzing various wound biomarkers, and a packaging layer for encapsulating the sensor array (Figure 1B, Figure S1, and S6-S9). The construction of the microfluidic and sensing modules utilizes flexible substrates such as polyethylene terephthalate (PET) and PI films, allowing the flexible patch containing the microfluidic and sensing modules to conform to different wound areas for effective sampling and analysis (Figure 1B-D and Figure S10). The sensing module employs advanced micro-nano-engineered carbon-based materials as electrode substrates, including silver nanodendrite-modified carbon dots/carbon nanotube nanocomposites (AgNDs/CDs-CNTs), CDs-CNTs nanocomposites, and laser-engraved graphene (LEG). These materials ensure sensitive and selective detection of O2, UA, and temperature in raw wound exudate (Figure 1E). The electronic module contains reusable printed circuit board (PCB) components for signal transduction, data processing, and wireless communication (Figure 1F, G). Electrochemical techniques, including current-time (i-t), differential pulse voltammetry (DPV), open circuit potential (OCP), and resistance-time (R-t), are employed to monitor biomarkers in wound exudate (Figure 1H). The integrated operation of the three modules ensures seamless functionality: the biomimetic microfluidic module effectively captures and transports wound exudate, the sensing module performs in situ multiplexed biomarker analysis, and the electronic module facilitates real-time wireless data transmission to the user’s device. 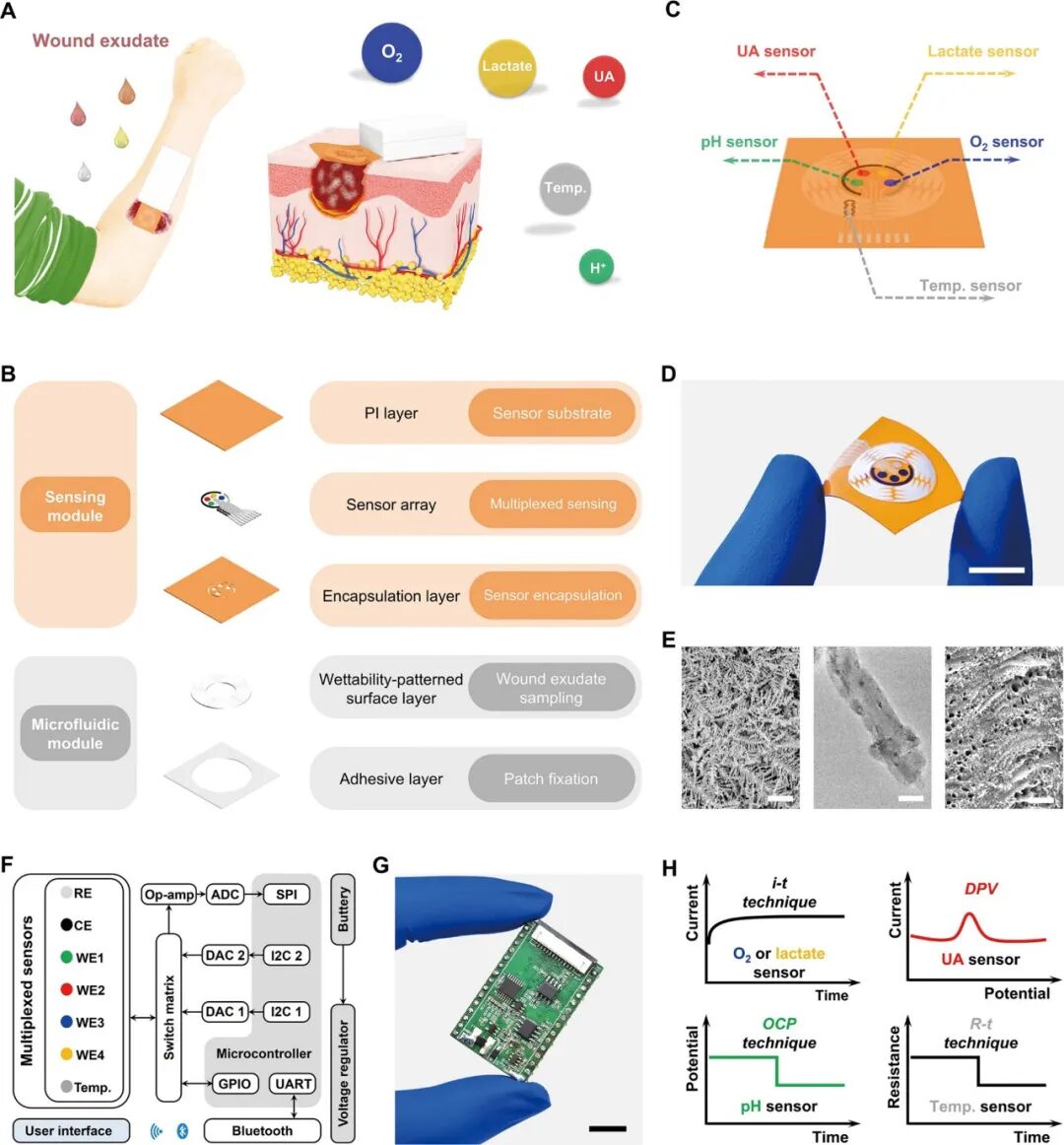 Figure 1. Schematic of FIWBMWT for in situ dynamic monitoring of wound exudate.Design and Characteristics of the Sensing ModuleFigure 2A shows the sensing module of FIWBMWT, which contains a sensor array for skin conformal and multiplexed analysis of wound exudate biomarkers, including O2, UA, lactate, pH, and temperature. The pH sensor is prepared by modifying the screen-printed electrode (SPE, Figure S7, S11, and S12) with a proton-selective membrane of polyaniline (PANI). PANI undergoes protonation and deprotonation reactions based on the pH of the surrounding solution, affecting the electrical properties (potential) of PANI (Figure 2B). By monitoring the changes in PANI potential, pH detection of the surrounding solution can be achieved. The proposed pH sensor shows a linear relationship between OCP and pH in the range of 4-9 (Figure 2C), matching the physiological pH range of wound exudate. The inherent selectivity of the proton-selective membrane ensures its superior specificity to proton ions compared to other commonly present ions in wound exudate (Figure S13). Additionally, the sensor exhibits stable responses within the physiological temperature range of wound exudate (Figure S14). To fabricate the temperature sensor, CO2 laser engraving was used to create serpentine line structures on flexible PI films, resulting in compact LEG (Figure 2E). As the temperature increases, the conductivity increases due to electron-phonon scattering and increased electron thermal velocity in the interlayer (Figure 2D). At the same time, the sensitivity of this LEG structure to strain changes is minimal (Figure 2F). The sensor exhibits a linear correlation between resistance changes and temperature changes in the range of 30-50 °C, with a sensitivity of approximately −0.056% °C–1 (Figure 2G and Figure 15), indicating high feasibility for in vivo temperature monitoring of wound exudate (Figure S16). Given that UA levels in wound exudate can be as low as 50 μM, developing a highly sensitive UA sensor is crucial. To this end, carbon-based nanomaterials with multidimensional structures, particularly CDs-CNT, were synthesized and used as electrode substrates due to their high electrochemical reactivity and unique structure (Figure 2H-J and Figure S17-S24). The ID/IG ratio of CDs-CNT reaches up to 0.548, indicating the presence of abundant defect sites that enhance electron transfer at the sensing interface and improve UA detection performance (Figure S19). Fourier-transform infrared (FT-IR) spectroscopy and X-ray photoelectron spectroscopy (XPS) confirmed the presence of oxygen-containing functional groups in CDs-CNT, which may enhance hydrophilicity and reduce charge transfer resistance at the sensing interface (Figure S20 and S21). Photoluminescence studies showed a significant quenching phenomenon after modifying CNT with CDs (Figure S22), indicating that strong electronic coupling accelerates electron transfer dynamics and enhances electrocatalytic activity. By modifying CDs-CNT on SPE (Figure S24), DPV results further demonstrate the excellent electrocatalytic activity of CDs-CNT for UA electrooxidation, with increased anodic current and reduced oxidation potential compared to SPE or CNT alone (Figure 2J). This is likely due to the unique structure of CDs-CNT and enhanced electron transfer improving UA electrocatalytic performance. The UA sensor exhibits a wide linear detection range (50-900 μM) with a low detection limit of 2.2 μM, covering physiological UA levels in wound exudate (Figure 2K). (4) Stability against temperature changes, common analytes, and real-time calibration through pH adjustments ensures accurate UA analysis (Figure S25-S27 and S36).
Figure 1. Schematic of FIWBMWT for in situ dynamic monitoring of wound exudate.Design and Characteristics of the Sensing ModuleFigure 2A shows the sensing module of FIWBMWT, which contains a sensor array for skin conformal and multiplexed analysis of wound exudate biomarkers, including O2, UA, lactate, pH, and temperature. The pH sensor is prepared by modifying the screen-printed electrode (SPE, Figure S7, S11, and S12) with a proton-selective membrane of polyaniline (PANI). PANI undergoes protonation and deprotonation reactions based on the pH of the surrounding solution, affecting the electrical properties (potential) of PANI (Figure 2B). By monitoring the changes in PANI potential, pH detection of the surrounding solution can be achieved. The proposed pH sensor shows a linear relationship between OCP and pH in the range of 4-9 (Figure 2C), matching the physiological pH range of wound exudate. The inherent selectivity of the proton-selective membrane ensures its superior specificity to proton ions compared to other commonly present ions in wound exudate (Figure S13). Additionally, the sensor exhibits stable responses within the physiological temperature range of wound exudate (Figure S14). To fabricate the temperature sensor, CO2 laser engraving was used to create serpentine line structures on flexible PI films, resulting in compact LEG (Figure 2E). As the temperature increases, the conductivity increases due to electron-phonon scattering and increased electron thermal velocity in the interlayer (Figure 2D). At the same time, the sensitivity of this LEG structure to strain changes is minimal (Figure 2F). The sensor exhibits a linear correlation between resistance changes and temperature changes in the range of 30-50 °C, with a sensitivity of approximately −0.056% °C–1 (Figure 2G and Figure 15), indicating high feasibility for in vivo temperature monitoring of wound exudate (Figure S16). Given that UA levels in wound exudate can be as low as 50 μM, developing a highly sensitive UA sensor is crucial. To this end, carbon-based nanomaterials with multidimensional structures, particularly CDs-CNT, were synthesized and used as electrode substrates due to their high electrochemical reactivity and unique structure (Figure 2H-J and Figure S17-S24). The ID/IG ratio of CDs-CNT reaches up to 0.548, indicating the presence of abundant defect sites that enhance electron transfer at the sensing interface and improve UA detection performance (Figure S19). Fourier-transform infrared (FT-IR) spectroscopy and X-ray photoelectron spectroscopy (XPS) confirmed the presence of oxygen-containing functional groups in CDs-CNT, which may enhance hydrophilicity and reduce charge transfer resistance at the sensing interface (Figure S20 and S21). Photoluminescence studies showed a significant quenching phenomenon after modifying CNT with CDs (Figure S22), indicating that strong electronic coupling accelerates electron transfer dynamics and enhances electrocatalytic activity. By modifying CDs-CNT on SPE (Figure S24), DPV results further demonstrate the excellent electrocatalytic activity of CDs-CNT for UA electrooxidation, with increased anodic current and reduced oxidation potential compared to SPE or CNT alone (Figure 2J). This is likely due to the unique structure of CDs-CNT and enhanced electron transfer improving UA electrocatalytic performance. The UA sensor exhibits a wide linear detection range (50-900 μM) with a low detection limit of 2.2 μM, covering physiological UA levels in wound exudate (Figure 2K). (4) Stability against temperature changes, common analytes, and real-time calibration through pH adjustments ensures accurate UA analysis (Figure S25-S27 and S36). 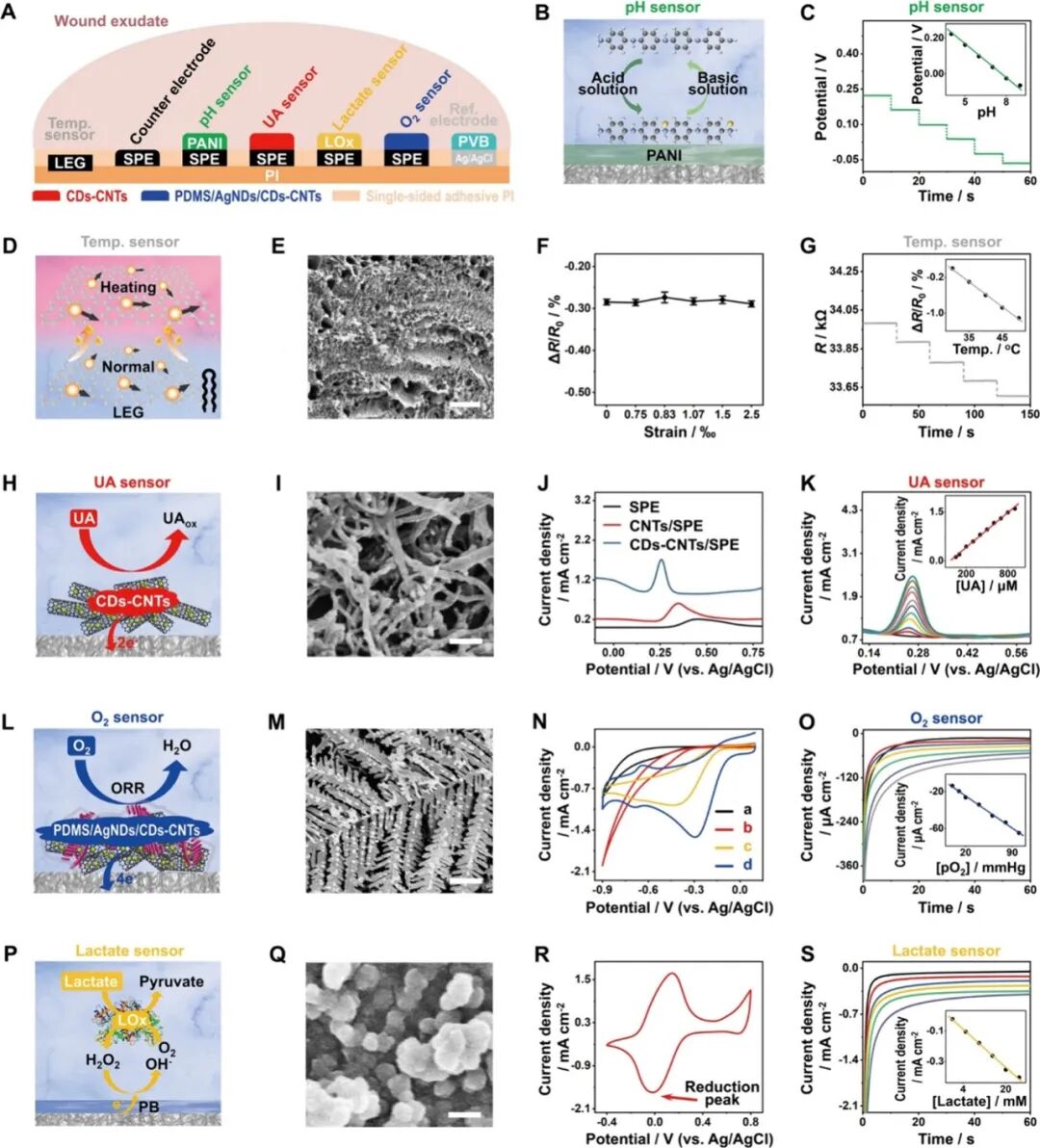 Figure 2. Design and Characteristics of the Sensing Module.Due to the complex environment and low concentrations (<10 mmHg), O2 sensing in raw wound exudate poses challenges. To address this, an O2 sensor (PDMS/AgNDs/CDs-CNTs/SPE) was prepared by electrodepositing silver nanodendrites (AgNDs)—an efficient O2 reduction electrocatalyst—onto CDs-CNTs, followed by applying a selective polydimethylsiloxane (PDMS) diffusion membrane (Figure 2L, M, and Figure S28). Compared to standalone CDs-CNT or AgNDs, the performance of AgNDs/CDs-CNT for O2 reduction reactions is significantly enhanced (Figure 2N and Figure S29), indicating a unique synergistic effect between Ag nanoparticles (such as AgNDs in this study) with excellent O2 reduction performance and CDs-CNT that facilitate electron transfer. PDMS allows O2 to permeate while blocking other biomolecules (such as H2O2), which is crucial for selectively monitoring O2 under physiological conditions. Using i-t techniques, the O2 sensor has a linear dynamic range of 5-100 mmHg, covering physiological wound exudate O2 levels (Figure 2O). The sensor exhibits high selectivity against interferences (Figure S30), stability under different pH and temperature conditions (Figure S31 and S32), and robustness during continuous monitoring, bending, and strain (Figure S37-S41). The measurements closely match those obtained using a dissolved oxygen meter, confirming its accuracy (Figure S36). Lactate was measured using an amperometric enzyme sensor with lactate oxidase (LOx) immobilized in a Prussian blue (PB)-chitosan film (Figure 2P-R). The lactate sensor detects levels between 1-30 mM, covering physiological lactate concentrations in wound exudate (Figure 2S). The inclusion of PB as an enzyme mediator in the lactate sensor allows for low-potential detection of H2O2 generated by enzyme-mediated lactate oxidation (Figure 2Q,R), effectively reducing interference from other components in wound exudate (Figure S33). However, the performance of the sensor is influenced by pH and temperature, reflecting the LOx activity dependence (Figure S34 and S35). Real-time calibration using integrated pH and temperature sensors ensures reliable lactate measurements (Figure S34–S36). The sensing module demonstrates a wide dynamic range, high selectivity, and excellent stability under continuous use, bending, and across production batches (Figure S37–S41). These features enable precise, in situ, dynamic assessment of O2, UA, lactate, pH, and temperature in wound exudate.
Figure 2. Design and Characteristics of the Sensing Module.Due to the complex environment and low concentrations (<10 mmHg), O2 sensing in raw wound exudate poses challenges. To address this, an O2 sensor (PDMS/AgNDs/CDs-CNTs/SPE) was prepared by electrodepositing silver nanodendrites (AgNDs)—an efficient O2 reduction electrocatalyst—onto CDs-CNTs, followed by applying a selective polydimethylsiloxane (PDMS) diffusion membrane (Figure 2L, M, and Figure S28). Compared to standalone CDs-CNT or AgNDs, the performance of AgNDs/CDs-CNT for O2 reduction reactions is significantly enhanced (Figure 2N and Figure S29), indicating a unique synergistic effect between Ag nanoparticles (such as AgNDs in this study) with excellent O2 reduction performance and CDs-CNT that facilitate electron transfer. PDMS allows O2 to permeate while blocking other biomolecules (such as H2O2), which is crucial for selectively monitoring O2 under physiological conditions. Using i-t techniques, the O2 sensor has a linear dynamic range of 5-100 mmHg, covering physiological wound exudate O2 levels (Figure 2O). The sensor exhibits high selectivity against interferences (Figure S30), stability under different pH and temperature conditions (Figure S31 and S32), and robustness during continuous monitoring, bending, and strain (Figure S37-S41). The measurements closely match those obtained using a dissolved oxygen meter, confirming its accuracy (Figure S36). Lactate was measured using an amperometric enzyme sensor with lactate oxidase (LOx) immobilized in a Prussian blue (PB)-chitosan film (Figure 2P-R). The lactate sensor detects levels between 1-30 mM, covering physiological lactate concentrations in wound exudate (Figure 2S). The inclusion of PB as an enzyme mediator in the lactate sensor allows for low-potential detection of H2O2 generated by enzyme-mediated lactate oxidation (Figure 2Q,R), effectively reducing interference from other components in wound exudate (Figure S33). However, the performance of the sensor is influenced by pH and temperature, reflecting the LOx activity dependence (Figure S34 and S35). Real-time calibration using integrated pH and temperature sensors ensures reliable lactate measurements (Figure S34–S36). The sensing module demonstrates a wide dynamic range, high selectivity, and excellent stability under continuous use, bending, and across production batches (Figure S37–S41). These features enable precise, in situ, dynamic assessment of O2, UA, lactate, pH, and temperature in wound exudate.
Design and Performance Characterization of the Microfluidic Module
Sweat and wound exudate are two important epidermal biological fluids in the human body. Unlike the rapid excessive secretion of sweat during short-term exercise, the secretion rate of wound exudate is relatively slow and in smaller volumes. Moreover, epidermal wounds are often irregularly shaped, making effective and skin-conforming collection of wound exudate a key prerequisite for dynamic, in situ monitoring. Inspired by the glandular hairs of the tomato (Solanum lycopersicum), which possess unique conical microstructures capable of spontaneously, directionally, and efficiently transporting droplets from the apex to the base (Figure 3A, B, Figure S42, and Movie S1), we designed a biomimetic microfluidic module. This module employs an asymmetric wedge pattern and super-contrast wettability surface engineering to achieve directional, efficient, and skin-conforming collection and transport of wound exudate (Figure 3C, D). The biomimetic microfluidic module is constructed on a flexible PET patch with patterned superhydrophilic and superhydrophobic regions (Figure 3C, D, and Figure S43–S45). In the superhydrophilic region, five individual sampling units are arranged around a central collection area (Figure 3C). Each sampling unit consists of a main wedge-shaped sampler connected to several secondary wedge-shaped samplers, with the tips of the samplers designed to face the opposite direction of the biological fluid transport direction (Figure 3C and Figure S5). To demonstrate the module’s performance in directional biological fluid collection, the delivery process of a single sampling unit to artificial wound exudate was recorded in real-time (Figure 3E-G and Movie S2). When a droplet of artificial wound exudate (containing 20 mM methyl violet, viscosity of 1.08 mPa·s) contacts the secondary wedge-shaped sampler, the exudate spontaneously flows directionally along the secondary and primary samplers, accumulating in the central collection area in approximately 0.55 seconds (Figure 3E and Movie S2). This result highlights the module’s efficient directional liquid transport capability. In contrast, when a symmetric rectangular sampler replaced the asymmetric wedge design, no significant directional transport was observed, likely due to the lack of driving force caused by the absence of a chemical wettability gradient (Figure 3F and Movie S3). Similarly, when the superhydrophilic region was removed, leaving only the superhydrophobic surface, effective liquid transport also disappeared (Figure 3G and Movie S4). These findings indicate that the asymmetric wedge pattern and super-contrast wettability are crucial for efficient, directional collection of artificial wound exudate. In addition to the previously mentioned artificial wound exudate with a viscosity of 1.08 mPa·s, the designed biomimetic microfluidic module can also spontaneously, directionally, and effectively transport artificial wound exudate with viscosities within the physiological range of wound exudate (Table S1 and Figure S46 and S47). These surface engineering strategies integrate to make the proposed biomimetic microfluidic module a promising candidate for precise, dynamic, and in situ analysis of wound exudate.  Figure 3. Design and Characteristics of the Microfluidic Module for Wound Exudate Collection.
Figure 3. Design and Characteristics of the Microfluidic Module for Wound Exudate Collection.
Validation of FIWBMWT for Analysis of Raw Wound Exudate
FIWBMWT can be directly applied to skin wound sites for in situ and dynamic analysis of various biomarkers in wound exudate (Figure 4A). Utilizing a full-thickness wound model in mice simulating human skin injuries, FIWBMWT demonstrated excellent biocompatibility (Figure S48), enabling dynamic, wireless simultaneous tracking of wound exudate O2, UA, lactate, pH, and temperature in both non-inoculated and inoculated bacterial wounds (Figure 4B). In non-inoculated wounds, the O2 levels in wound exudate initially decreased but then showed an upward trend as the wound healed. In contrast, UA, lactate, pH, and temperature levels initially increased and then gradually decreased over time (Figure 4C and Figure S49). The initial decrease in O2 levels may be due to vascular damage impairing blood circulation at the wound site. The increase in uric acid and lactate levels can be attributed to cells relying on glycolysis for energy in a hypoxic environment. Additionally, the increase in pH and temperature may be related to inflammation caused by vascular damage (Figure 4D). As angiogenesis progresses during the wound healing process, O2 levels gradually return to baseline, while uric acid, lactate, pH, and temperature decrease, reflecting the restoration of aerobic metabolism and reduction of inflammation. In inoculated wounds, the trends of O2, uric acid, lactate, pH, and temperature levels were similar to those observed in non-inoculated wounds, but with significant differences in magnitude. Between 12 to 72 hours, inoculated wounds exhibited lower O2 levels and higher UA, lactate, pH, and temperature levels compared to non-inoculated wounds (Figure 4C and Figure S49). The lower O2 levels in inoculated wounds may be due to more severe vascular damage and blood flow interruption caused by bacterial-induced inflammatory responses (Figure 4D). Hypoxia exacerbates cellular reliance on glycolysis, further elevating UA and lactate levels. Furthermore, wound hypoxia and bacterial infection enhance inflammatory responses, leading to increased pH and temperature. The presence of anaerobic bacteria thriving under hypoxic conditions produces alkaline substances, further elevating the pH of wound exudate (Figure 4D). Comparative analysis of O2, UA, lactate, pH, and temperature levels in infected and non-infected wounds revealed statistically significant differences in measurements obtained through FIWBMWT (Figure 4G and Tables S2 and S3). This may also explain why infected wounds exhibited lower O2 levels and higher UA, lactate, pH, and temperature levels compared to non-infected wounds 72 hours after wound formation (Figure 4C and Figure S49), with more severe inflammation (Figure 4E, F). Therefore, the proposed FIWBMWT provides a promising and user-friendly tool for assessing wound conditions by monitoring wound exudate, O2, UA, lactate, pH, and temperature (Table S4). Statistical analysis further shows a strong correlation between UA, lactate, pH, or temperature levels and O2 content in wound exudate (Figure 4H). These correlations are consistent with physiological responses under different wound conditions (i.e., infected and non-infected wounds): (a) hypoxia alters cellular metabolism, increasing UA and lactate levels; (b) hypoxia exacerbates inflammation, elevating pH and temperature; and (c) hypoxia promotes anaerobic bacterial colonization, producing alkaline substances that raise pH levels. These observations emphasize that O2 is a key factor influencing wound biomarkers and suggest that increasing O2 levels in infected wounds may reduce infection-related biomarker levels, thereby promoting faster wound healing. Therefore, strategies aimed at modulating O2 at the wound site may represent an effective wound management approach. 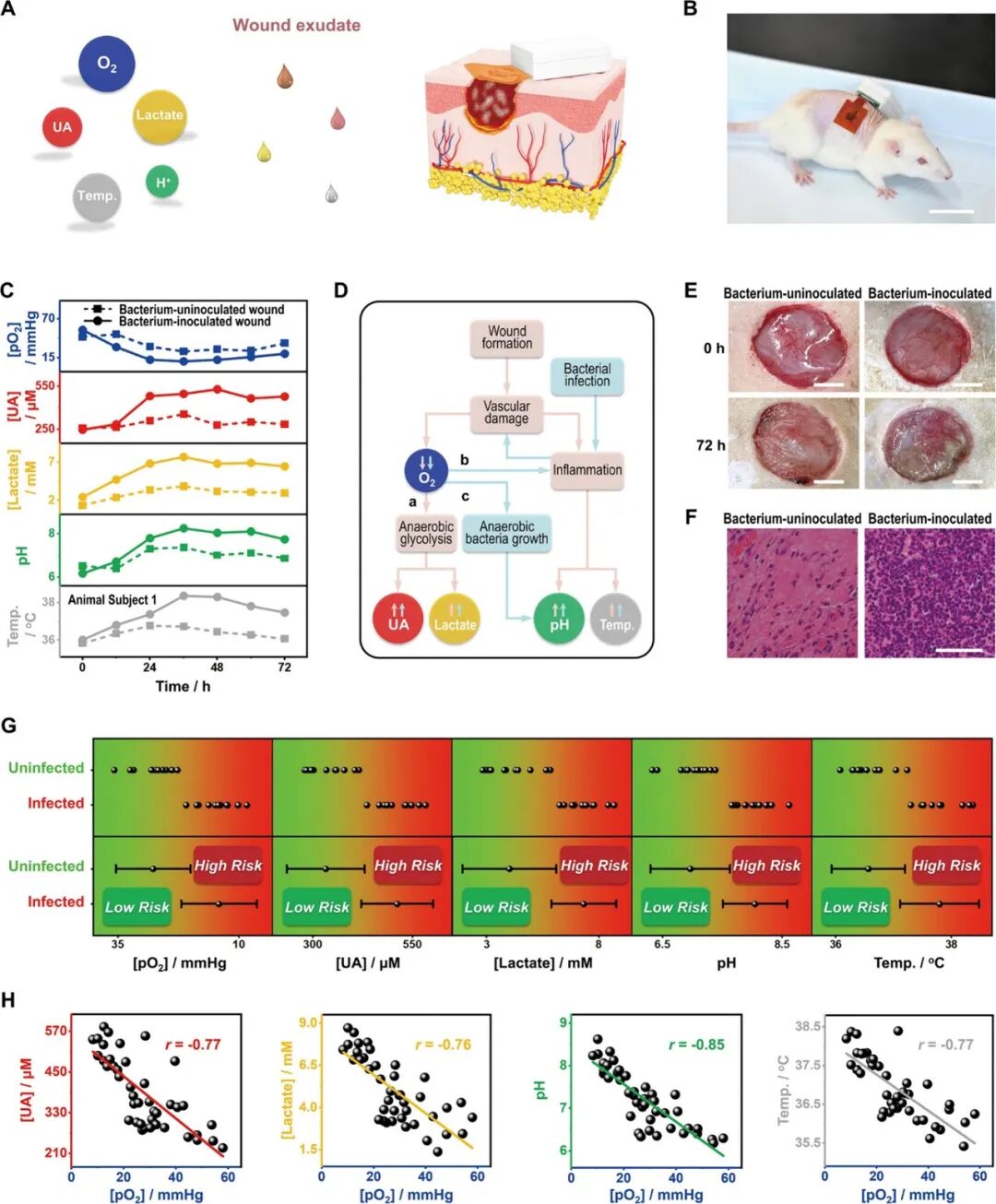 Figure 4. Validation of FIWBMWT for In Situ Analysis of Epidermal Wound Exudate.
Figure 4. Validation of FIWBMWT for In Situ Analysis of Epidermal Wound Exudate.
Potential Wound Management Assessment with FIWBMWT
Wounds are an inevitable part of daily life. Improper or untimely wound management can lead to delayed healing, uncontrolled infections, and even life-threatening complications. For individuals with specific conditions such as diabetes, wound healing is often impaired, with significantly longer healing times compared to healthy individuals. This highlights the urgent need for personalized wound management strategies for such high-risk populations. Unlike previously reported wearable sensors that primarily target “normal” subjects for wound exudate analysis, this study specifically selected diabetic subjects to validate the wound monitoring and management capabilities of FIWBMWT. In diabetic patients, hyperglycemia can lead to narrowing or blockage of capillaries around the wound, affecting the transport of nutrients and oxygen, thereby slowing the formation of new blood vessels in the wound tissue, significantly delaying wound healing and increasing the risk of infection. To simulate this condition, we employed a diabetic mouse model (Figure 5A). After skin injury and subsequent bacterial inoculation, the biomarkers in wound exudate—uric acid (UA), lactate, pH, and temperature—gradually increased on the first day, while O2 levels decreased (Figure 5B and Figure S50). By assessing these biomarkers and comparing them with the wound risk assessment criteria established in Figure 4G and Table S4, FIWBMWT successfully detected wound infections as early as the first day, outperforming traditional visual observations, which typically detect infections on the second day (Figure 5C). To promote wound healing, local hyperbaric oxygen therapy (LHBOT, twice daily) was administered to the wounds (Figure 5A). Over time, the levels of biomarkers in wound exudate transitioned from the “high wound infection risk” range ([pO2] < 20 mmHg, [UA] > 431 μM, [lactate] > 6.1 mM, pH > 7.6, temperature > 37.2 °C) to the “low wound infection risk” range ([pO2] > 22 mmHg, [UA] < 420 μM, [lactate] < 5.9 mM, pH < 7.5, temperature <37.1 °C) (Figure 5B and 4G and Table S4). Simultaneously, the progress of wound healing was clearly visible from wound photographs (Figure 5C). In contrast, wounds that did not receive additional LHBOT treatment exhibited prolonged “high wound infection risk” status and slower healing progress. These results were confirmed through analysis of wound exudate biomarkers, wound photographs, and histological assessments of wound tissue (Figure 5B-D). These control experiments highlight the advantages of FIWBMWT in monitoring wound exudate biomarkers, aiding in the management of wounds in high-risk populations such as diabetics. Given the limited sample size in this study, future research integrating machine learning, artificial intelligence, and big data analysis is expected to further enhance the accuracy and scalability of personalized wound management systems.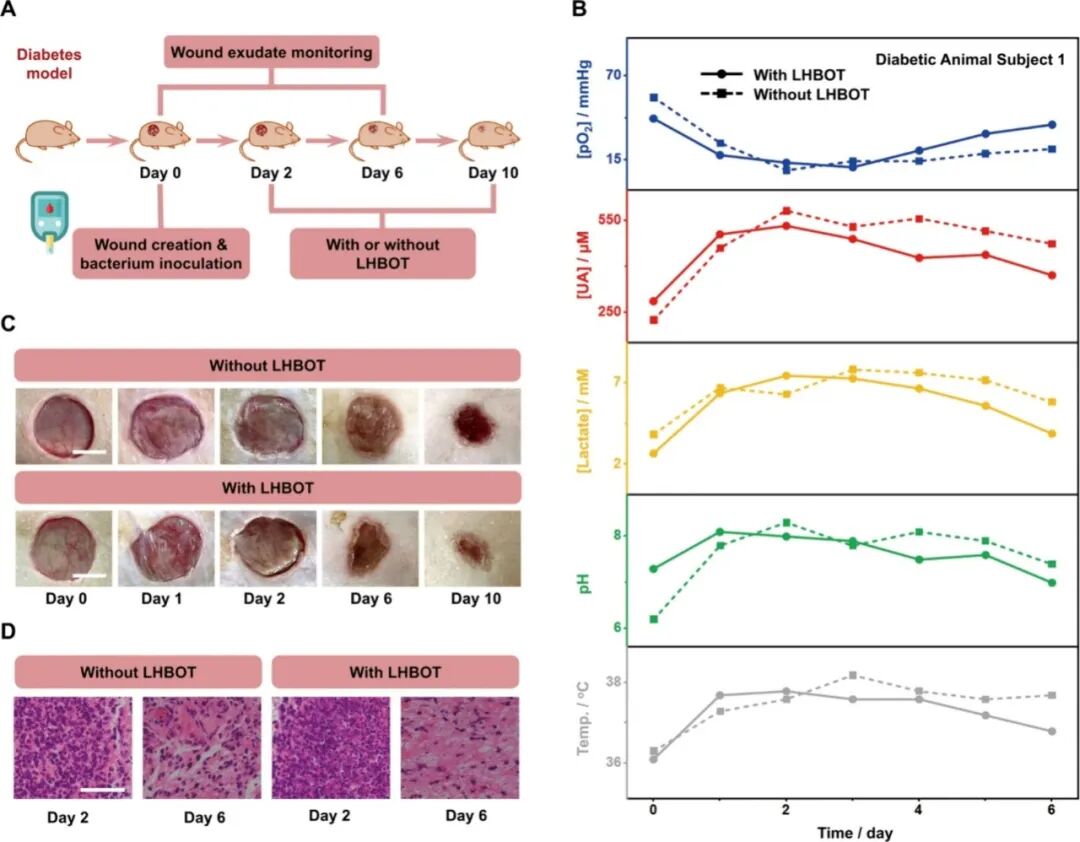 Figure 5. Assessment of Potential Wound Management Using FIWBMWT.ConclusionThe authors developed the first fully integrated wearable biomimetic microfluidic wound tracker (FIWBMWT), capable of non-invasive, skin-adhered, dynamic monitoring of wound exudate O2 and other key biomarkers, including UA, lactate, pH, and temperature, for potential applications in wound management. FIWBMWT is characterized by its comprehensive integration, enabling seamless sampling, monitoring, and wireless data transmission, offering superior practicality compared to traditional devices with limited modularity. Inspired by the directional fluid transport characteristics of glandular hairs of the tomato (Solanum lycopersicum), the biomimetic microfluidic module effectively facilitates the directional and efficient collection of wound exudate, laying a solid foundation for reliable analysis. As the core of the sensing module, the O2 sensor utilizes the synergistic properties of CDs-CNT nanocomposites, AgND, and PDMS membranes to achieve high sensitivity, selectivity, and dynamic in situ O2 measurements in raw wound exudate. Furthermore, the multidimensional layered architecture and high defect characteristics of the CDs-CNT-based UA sensor exhibit excellent electrochemical reactivity, enabling precise and sensitive quantification of UA in wound exudate. FIWBMWT can simultaneously measure O2, UA, lactate, pH, and temperature, providing a comprehensive understanding of wound status that cannot be achieved by monitoring individual biomarkers alone. This synchronous monitoring capability offers comprehensive insights into the wound microenvironment, allowing for detailed assessments of dynamic changes associated with wound healing and infection. Validation studies conducted using a diabetic mouse model simulating high-risk wound conditions in diabetic patients confirmed that FIWBMWT can correlate O2 levels during the wound healing process with UA, lactate, pH, and temperature. These findings emphasize the potential of FIWBMWT in personalized wound assessment and management, particularly for populations at higher risk of delayed healing and infection, such as diabetic patients. By integrating advanced microfluidic and sensing technologies with real-time wireless data transmission, FIWBMWT represents a powerful and versatile platform for personalized wound monitoring and customized treatment interventions, paving the way for advancements in future wound care strategies.References:A Fully Integrated Wearable Biomimetic Microfluidic Wound Tracker for In Situ Dynamic Monitoring of Wound Exudate Oxygen, Wei Deng, Mimi Sun, Mengzhu Cao, Chong-Bo Ma, Xiangjie Bo, Jing Bai, and Ming Zhou, ACS Nano Article ASAP, DOI: 10.1021/acsnano.5c04304
Figure 5. Assessment of Potential Wound Management Using FIWBMWT.ConclusionThe authors developed the first fully integrated wearable biomimetic microfluidic wound tracker (FIWBMWT), capable of non-invasive, skin-adhered, dynamic monitoring of wound exudate O2 and other key biomarkers, including UA, lactate, pH, and temperature, for potential applications in wound management. FIWBMWT is characterized by its comprehensive integration, enabling seamless sampling, monitoring, and wireless data transmission, offering superior practicality compared to traditional devices with limited modularity. Inspired by the directional fluid transport characteristics of glandular hairs of the tomato (Solanum lycopersicum), the biomimetic microfluidic module effectively facilitates the directional and efficient collection of wound exudate, laying a solid foundation for reliable analysis. As the core of the sensing module, the O2 sensor utilizes the synergistic properties of CDs-CNT nanocomposites, AgND, and PDMS membranes to achieve high sensitivity, selectivity, and dynamic in situ O2 measurements in raw wound exudate. Furthermore, the multidimensional layered architecture and high defect characteristics of the CDs-CNT-based UA sensor exhibit excellent electrochemical reactivity, enabling precise and sensitive quantification of UA in wound exudate. FIWBMWT can simultaneously measure O2, UA, lactate, pH, and temperature, providing a comprehensive understanding of wound status that cannot be achieved by monitoring individual biomarkers alone. This synchronous monitoring capability offers comprehensive insights into the wound microenvironment, allowing for detailed assessments of dynamic changes associated with wound healing and infection. Validation studies conducted using a diabetic mouse model simulating high-risk wound conditions in diabetic patients confirmed that FIWBMWT can correlate O2 levels during the wound healing process with UA, lactate, pH, and temperature. These findings emphasize the potential of FIWBMWT in personalized wound assessment and management, particularly for populations at higher risk of delayed healing and infection, such as diabetic patients. By integrating advanced microfluidic and sensing technologies with real-time wireless data transmission, FIWBMWT represents a powerful and versatile platform for personalized wound monitoring and customized treatment interventions, paving the way for advancements in future wound care strategies.References:A Fully Integrated Wearable Biomimetic Microfluidic Wound Tracker for In Situ Dynamic Monitoring of Wound Exudate Oxygen, Wei Deng, Mimi Sun, Mengzhu Cao, Chong-Bo Ma, Xiangjie Bo, Jing Bai, and Ming Zhou, ACS Nano Article ASAP, DOI: 10.1021/acsnano.5c04304