
Table of Contents/contents/
> Metabolic Characteristics of Critically Ill Patients
> Individualized Application of Vitamins
> Monitoring and Supplementation of Trace Elements
> Disease-Specific Adjustment Strategies
High Metabolic State and Nutritional Impact in Critical Illness
Oxidative Stress and Vitamin Requirements
> Critically ill patients exhibit intense inflammatory responses, significantly increasing oxidative stress, leading to a substantial rise in the demand for antioxidant vitamins such as Vitamin C and E to maintain redox balance in the body.
> Studies have shown that Vitamin C levels in septic patients can drop to one-tenth of normal values, and timely supplementation can improve prognosis and reduce infection risk.
Disruption of Trace Element Metabolism
> Trace elements such as zinc and selenium play critical roles in immune function and antioxidant defense. Critically ill patients often experience deficiencies due to factors like intestinal absorption disorders and inflammatory consumption.
> A survey of burn patients indicated that early post-injury serum zinc levels can decrease by 30%-50%, and zinc supplementation can accelerate wound healing and reduce infection rates.
Impact of Medications on Nutrient Absorption
> Antibiotics can disrupt gut microbiota, affecting the synthesis of Vitamin K and B vitamins; glucocorticoids can inhibit the activation of Vitamin D, increasing calcium excretion and leading to osteoporosis risk.
> Long-term use of proton pump inhibitors can reduce gastric acid secretion, affecting the absorption of iron, Vitamin B12, and other nutrients, necessitating regular monitoring of related indicators and adjustment of supplementation plans.
Micronutrients (MNs)
Trace elements and vitamins, collectively referred to as micronutrients (MNs), are essential for human metabolism.
Recent research has highlighted the importance of MNs in common pathologies, with significant deficiencies adversely affecting outcomes.
However, specific knowledge remains limited among clinicians, with trace elements being even less recognized than vitamins.
There are two levels of concern: (1) public health, and (2) individual health. Deficiencies in iodine, iron, vitamin A, and zinc are among the world’s most serious health risk factors.
Causes of Micronutrient Deficiency
Micronutrient deficiencies are often associated with three categories of factors:
> Insufficient intake or utilization barriers (e.g., dietary imbalance, malabsorption syndromes, alcoholism, chronic liver disease, renal dysfunction/failure, acid-suppressing medications)
> Increased loss or consumption (e.g., extensive burns, prolonged inflammation and oxidative stress, polyuria, CRRT, gastrointestinal fistulas)
> Drug-related effects (e.g., isoniazid, phenobarbital, phenytoin, theophylline, tricyclic antidepressants)
Factors Influencing Micronutrient Deficiency in Critical Illness
> Possible causes before ICU admission (e.g., reduced dietary intake, prolonged hospitalization, alcoholism)
> Treatment measures in the ICU (e.g., RRT, enteral feeding, frequent blood draws)
> Disease impacts (e.g., gastrointestinal diseases, enteric fistulas, bile loss, renal failure, liver failure) leading to micronutrient deficiencies
> Micronutrient deficiencies can cause cellular metabolic abnormalities, associated with encephalopathy, myopathy and neuropathy, stomatitis, dermatitis, delayed wound healing, low cardiac output, and recurrent infections.
Insufficient Nutritional Intake in Critically Ill Patients
Research
A prospective cohort study measured serum micronutrient levels in ICU patients during the first week of admission, including selenium, beta-carotene, Vitamin C, Vitamin E, Vitamin B1, and B6, and compared them with micronutrient levels in age-matched healthy controls.
The study explored the correlation between micronutrients in ICU patients and disease severity, inflammation, and intestinal micronutrient intake.
> Selenium, Vitamin A, Vitamin C, and Vitamin E levels in ICU patients were significantly lower than those in the control group (P<0.001)
> No significant differences were observed in Vitamin B1 and B6 levels between ICU patients and the control group.
> Vitamin C levels remained below normal values, significantly decreasing from day 1 to day 5 (P<0.01)
> Vitamin E levels also remained below normal values but significantly increased from day 5 to day 7 (p<0.01)
Interventions in Critical Care: Potential for Micronutrient Deficiency
In clinical practice, treatments such as CRRT, diuretics, inhibitors, antitumor therapies, and immunotherapies can affect trace elements, leading to deficiencies.
Drug Interactions: Impact on Micronutrients
Warfarin and Vitamin K Interaction
Patients on warfarin should avoid high doses of Vitamin K (from green leafy vegetables or supplements) to prevent interference with anticoagulation effects and increased thrombotic risk. Monitor the International Normalized Ratio (INR) and adjust warfarin dosage based on INR to avoid excessive or insufficient Vitamin K intake leading to anticoagulation failure.
Effects of Proton Pump Inhibitors
Long-term use of proton pump inhibitors (PPIs) can suppress gastric acid secretion, affecting the absorption of iron, Vitamin B12, magnesium, and other nutrients, necessitating supplementation of these nutrients. Regular monitoring of complete blood counts and magnesium levels is required to avoid nutrient deficiencies.
Monitoring and Adjusting Drug Interactions
Regular monitoring of blood drug concentrations and related indicators is essential, adjusting nutrient supplementation doses and methods based on drug interactions to avoid adverse reactions. By appropriately adjusting the use of medications and nutrients, therapeutic efficacy can be improved, and the incidence of complications reduced, requiring dynamic monitoring and adjustments based on the patient’s condition.
Research
Critically ill patients frequently present low micronutrient blood levels, which have been associated with illness severity and poor clinical outcomes; nevertheless, low levels do not always reflect deficiency.
The impact of the omnipresent inflammation, which causes redistribution of micronutrients between organs, must be integrated to enable interpretation of blood levels, and for some micronutrients, the interpretation requires assistance by biomarkers.
Research
Micronutrients are essential in the ICU. Due to their antioxidant properties and the high prevalence of low blood concentrations suggestive of deficiency, several large-scale RCTs have been conducted with variable success.
Micronutrient deficiencies are common in critically ill patients, and multiple RCT studies have been conducted, but results are inconsistent.
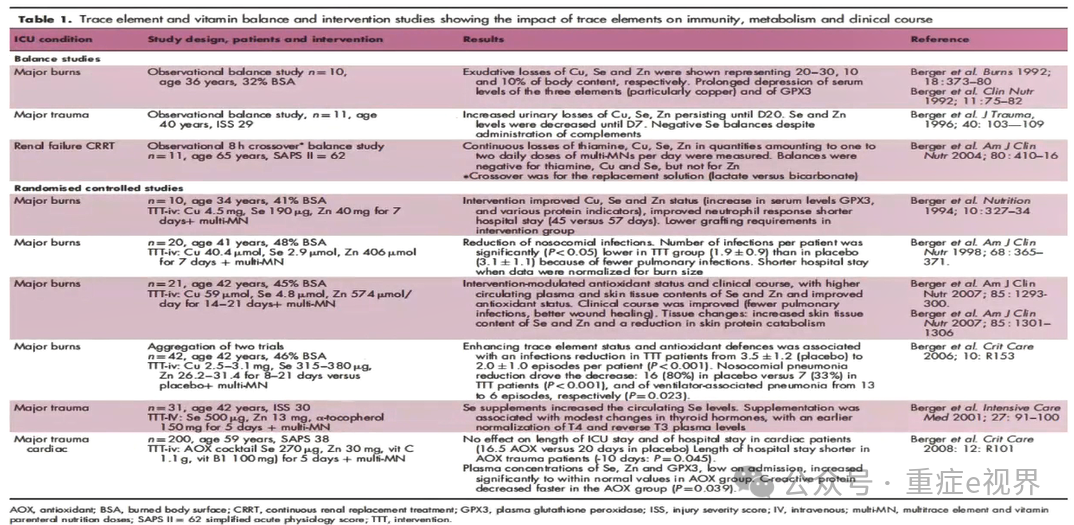
1
B Vitamins
Increased B Vitamin Requirements in Mechanically Ventilated Patients
Patients on mechanical ventilation experience increased energy expenditure, and Vitamin B1 deficiency can lead to lactic acidosis, affecting cardiopulmonary function; Vitamin B6 is involved in amino acid metabolism, and deficiency can lead to skin lesions and anemia.
Recommended intravenous supplementation of Vitamin B1 is 200-500 mg/d, and Vitamin B6 is 50-100 mg/d, which can improve mitochondrial function and promote energy metabolism.
Supplementation Plans for Long-term Parenteral Nutrition
Patients on long-term parenteral nutrition often have limited intestinal absorption, making them prone to B vitamin deficiencies. Supplementation of Vitamin B1, B6, and B12 is necessary to prevent neuropathy and anemia. It is recommended to supplement Vitamin B1 at 50-100 mg/d, Vitamin B6 at 20-50 mg/d, and Vitamin B12 at 100-200 μg, with regular monitoring of complete blood counts and neurological function indicators.
Adjustment of B Vitamins in Septic Patients
Septic patients experience metabolic disturbances, and B vitamins play a crucial role in energy metabolism and antioxidant defense, necessitating appropriate dose increases. Vitamin B1 can be increased to 200-500 mg/d intravenously, and Vitamin B6 to 50-100 mg/d, which helps improve mitochondrial function and reduce oxidative stress damage.
Vitamin B1 (Thiamine)
Vitamin B1 is a key cofactor in substrate oxidation. Therefore, Vitamin B1 deficiency can affect heart and brain function (Wernicke and Korsakoff syndromes).
Causes of deficiency: diuretics, continuous renal replacement therapy (CRRT), insufficient intake, and low body reserves.
Prevention and Treatment Strategies:Monitor and supplement phosphate to prevent “refeeding syndrome”; early nutritional management in critical illness; provide adequate thiamine (>100 mg/day).
2
Micronutrient Deficiency in Critically Ill Patients: Vitamin C
Vitamin C (ascorbic acid): Vitamin C plays an important role as an antioxidant and cofactor for various enzymes in the body.
Causes of deficiency: reduced intake, increased oxidative stress in the body (especially during sepsis).
Consequences of deficiency: endothelial cell damage, anemia, scurvy.
Vitamin C treatment (10 g/d for 4 consecutive days) reduces oxidative stress and inflammation levels in ARDS patients.
3
Micronutrient Deficiency in Critically Ill Patients: Carnitine
Carnitine (L-carnitine, or Vitamin BT)
Carnitine is an important mediator in mitochondrial biochemical reactions, playing a significant role in cellular energy metabolism. Critically ill patients often exhibit mitochondrial dysfunction, and carnitine deficiency is one of the causes. Carnitine deficiency can lead to unexplained muscle weakness, unexplained heart failure, and hyperlipidemia.
Causes of deficiency: liver and kidney dysfunction, reduced intake of amino acids and Vitamin C, patients undergoing CRRT.
Prevention and Treatment Strategies:Supplement carnitine, such as L-carnitine injection, with systemic application of 0.5-1 g/d, but excessive supplementation can lead to toxicity.
4
Micronutrient Deficiency in Critically Ill Patients: Vitamin D
Vitamin D
Vitamin D influences gene transcription in organs and tissues, with effects extending beyond calcium homeostasis and bone integrity, impacting the cardiovascular system and immune function. At ICU admission, over 50% of critically ill patients have low Vitamin D levels, with 24% having very low levels, which are associated with clinical prognosis.
Causes of deficiency: reduced intake, decreased renal conversion, decreased hepatic production, reduced sunlight exposure or intake, and effects of dialysis, ECMO, and plasma exchange.
Consequences of deficiency: increased risk of sepsis, ARDS, AKI, and increased mortality rates.
Prevention and Treatment Strategies:
> Routine supplementation: children 400-600 IU/d, adults 600-800 IU/d
> AKI, malabsorption syndrome, and cardiopulmonary bypass patients: 1000-4000 IU/d
> In the initial phase of critical illness, using loading doses can rapidly restore Vitamin D levels, followed by routine dosing.
5
Micronutrient Deficiency in Critically Ill Patients: Vitamin B12 and Folate
Vitamin B12 and Folate
Vitamin B12 is essential for DNA synthesis and nutrient metabolism. Folate is a cofactor for carbon transfer, crucial for nucleic acid and amino acid synthesis.
Causes of Vitamin B12 deficiency: gastric surgery, small bowel nutrition, and use of proton pump inhibitors.
Causes of folate deficiency: alcoholism; hemolysis, malignancies; drug use: methotrexate or trimethoprim; CRRT.
Consequences of deficiency: anemia, thrombocytopenia, leukopenia (folate deficiency), unexplained cognitive changes, or clinical evidence suggesting demyelination symptoms.
Prevention and Treatment Strategies: Clinical and laboratory diagnosis can be challenging in cases of deficiency, and preventive administration is recommended.
Micronutrient Deficiency and Disease Risk
Deficiencies in trace elements can lead to complications and even impact the prognosis of critically ill patients. Conversely, some diseases can also lead to micronutrient deficiencies.
Micronutrient Deficiency—Individualized Treatment
6
Zinc
Clinical Manifestations and Monitoring of Zinc Deficiency
Zinc deficiency can lead to symptoms such as taste disturbances, dry skin, delayed wound healing, and decreased immune function, affecting patient recovery. Regular monitoring of plasma zinc levels is necessary, with normal values ranging from 70-120 μg/dL, adjusting supplementation doses based on clinical manifestations to avoid excess or deficiency.
Zinc Interactions with Other Nutrients
Zinc has antagonistic effects with trace elements such as copper and iron; excessive zinc can inhibit copper absorption and affect iron utilization, necessitating balanced supplementation. When supplementing zinc, appropriate copper supplementation should be provided to maintain a copper/zinc ratio of 1:10-15, avoiding the occurrence of iron deficiency anemia.
Zinc’s indications and supplementation doses: Zinc is a key element for the immune system and wound healing; patients with trauma, enteric fistulas, and diarrhea experience increased intestinal losses and require timely supplementation. In the acute phase, intravenous supplementation of 15-30 mg/d can promote wound healing and enhance immune function, but long-term high doses should be monitored to prevent copper absorption inhibition, requiring monitoring of the copper/zinc ratio.
7
Selenium
The Importance of Selenium for Critically Ill Patients
Selenium is a crucial component of antioxidant enzymes; oxidative stress is significant in septic and ARDS patients, and selenium deficiency can exacerbate inflammatory responses and organ damage. An initial intravenous dose of 1000 μg, followed by 200-400 μg/d, can reduce mortality and improve prognosis, but long-term high doses may lead to selenium toxicity.
Association of Selenium with Other Diseases
Selenium deficiency in patients with thyroid storm can exacerbate the conversion disorder of T4 to T3, affecting thyroid function, necessitating appropriate supplementation. Caution is required regarding interactions with other medications, as selenium has a synergistic effect with Vitamin E, which can be used together to enhance antioxidant effects.
Clinical Manifestations and Monitoring of Selenium Deficiency
Selenium deficiency can lead to symptoms such as cardiomyopathy, myopathy, and decreased immune function, impacting patient safety; close monitoring is required. Regular testing of blood selenium levels is necessary, with normal values ranging from 70-130 μg/L, assessing the degree of selenium deficiency based on clinical symptoms and timely adjusting supplementation plans.
8
Iron
Clinical Manifestations and Monitoring of Iron Deficiency
Iron deficiency can lead to anemia, fatigue, and decreased immunity, affecting patient quality of life, necessitating regular monitoring. Regular testing of complete blood counts, ferritin, transferrin saturation, and other indicators is required, adjusting supplementation doses based on clinical manifestations to determine the degree of iron deficiency.
Principles and Limitations of Iron Supplementation
Iron is an important element for hematopoiesis and immune function, but supplementation during acute infection can exacerbate oxidative damage, requiring caution. Supplementation should only be considered when ferritin <100 μg/L and transferrin saturation <20%, avoiding blind iron supplementation that may lead to adverse reactions.
Relationship of Iron with Other Nutrients
Iron interacts synergistically with Vitamin C and B vitamins, promoting iron absorption and utilization, necessitating comprehensive supplementation. When supplementing iron, appropriate Vitamin C supplementation can enhance iron absorption rates, avoiding simultaneous supplementation with calcium, zinc, and other elements to prevent antagonistic effects.
Sepsis/Infectious Shock
Adjustment of Nutritional Therapy Plans
Patients with sepsis experience a hypermetabolic state, requiring high doses of Vitamin C (3 g/d) combined with hydrocortisone for synergistic anti-inflammatory effects, along with selenium supplementation to improve prognosis. Studies have shown that the combined use of Vitamin C and hydrocortisone can significantly reduce inflammatory markers in septic patients, shorten mechanical ventilation time, and reduce hospital stay.
Mechanisms of Action of Key Nutrients
Vitamin C has antioxidant and immune-regulating effects, reducing inflammatory responses and protecting cells from oxidative damage; hydrocortisone can inhibit the release of inflammatory mediators and stabilize cell membranes. Selenium can enhance antioxidant enzyme activity, reducing mortality in septic patients and improving organ dysfunction, requiring dynamic adjustment of doses based on the patient’s condition.
Clinical Application Cases and Effects
A tertiary hospital treated 50 septic patients using the above protocol, showing significant reductions in inflammatory markers (such as CRP, PCT), with an average mechanical ventilation time shortened by 3 days and hospital stay reduced by 5 days. This protocol has shown significant effects in clinical applications, but monitoring of blood glucose, electrolytes, and other indicators is necessary to avoid drug interactions and adverse reactions.
Acute Kidney Injury (AKI)
Adjustment of Nutritional Therapy Plans
Patients with AKI should avoid excessive Vitamin A (due to accumulation toxicity) and limit potassium and phosphorus (such as phosphate preparations) to avoid increasing renal burden. It is recommended to use low-potassium, low-phosphorus enteral nutrition formulations, adjusting the doses of Vitamin D, B vitamins, and others based on renal function.
Mechanisms of Action of Key Nutrients
Vitamin A is metabolized to retinol in the body; excessive amounts can lead to accumulation toxicity, affecting renal function; potassium and phosphorus metabolism disorders can exacerbate electrolyte imbalances and acidosis in AKI patients. Appropriately limiting the intake of these nutrients can reduce renal burden and promote renal function recovery, requiring dynamic monitoring of related indicators based on the patient’s condition.
Clinical Application Cases and Effects
A hospital treated 30 AKI patients using an individualized nutritional therapy plan, showing good control of blood potassium and phosphorus levels, with a reduction in renal function recovery time by 2 weeks and a reduction in hospital stay by 3 weeks. This protocol has shown significant effects in clinical applications, but monitoring of complete blood counts, renal function, and other indicators is necessary to avoid nutrient deficiencies or excesses leading to adverse reactions.
Liver Failure
Adjustment of Nutritional Therapy Plans
Patients with liver failure should avoid manganese (due to biliary excretion disorders), and Vitamin K at 110 mg/d can improve coagulation, requiring exclusion of portal vein thrombosis. It is recommended to use low-manganese enteral nutrition formulations, adjusting the dose of Vitamin K1 based on coagulation function to avoid bleeding risks.
Mechanisms of Action of Key Nutrients
Patients with liver failure have reduced bile secretion, affecting the absorption and metabolism of fat-soluble vitamins (such as Vitamins A, D, E, K); manganese can accumulate in the body due to biliary excretion disorders, leading to toxicity. Appropriately adjusting the intake of these nutrients can improve coagulation function and nutritional status in patients with liver failure, requiring dynamic monitoring of related indicators based on the patient’s condition.
Clinical Application Cases and Effects
A hospital treated 20 patients with liver failure using an individualized nutritional therapy plan, showing significant improvements in coagulation function, reduced bleeding risks, and a reduction in hospital stay by 1 week. This protocol has shown significant effects in clinical applications, but monitoring of liver function, coagulation function, and other indicators is necessary to avoid nutrient deficiencies or excesses leading to adverse reactions.
Research
To enable substrate metabolism, micronutrients (i.e., trace elements and vitamins) should be provided daily with parenteral nutrition (PN). Grade of recommendation: B-strong consensus (100% agreement).
The ESPEN guidelines recommend the provision of a combination of antioxidant micronutrients “in safe doses” (i.e., 5-10 times Dietary Reference Intakes=DRI).
Research
Individualized Nutritional Therapy in Critical Illness: 10 Expert Recommendations.
Individualized Monitoring of Micronutrients
Question: How should we personalize monitoring of micronutrient and vitamin deficiencies?
> Testing should be initiated after 6-7 days in ICU.
> Patients at risk of deficiencies are those with active depletion, especially on CRRT, known to lead to significant losses/low measured levels of multiple micronutrients and water-soluble vitamins in ~90% of patients within 5-7 days on CRRT.
> Additionally, intestinal losses, major drains, and major burns lead to MN deficiencies.
> Inflammation, generally present in ICU, complicates interpretation of results: in CRP>40 mg/l, some MNs will be below reference values not necessarily reflecting deficiency, with the exception of copper, which increases with inflammation.
Individualized Treatment of Micronutrients
Question: How should we personalize repletion of micronutrient and vitamin deficiencies?
> Which MNs are at risk? Among trace elements, those with identified clinical consequences in case of deficiency are copper, selenium, zinc, and iron.
> Values 20% below the laboratory’s reference value should raise concern for MN status and trigger repletion with PN multi-trace element/vitamins or administration of repletion doses if lower.
> When repletion is initiated, monitoring results is required at ~7-10 days.
Take Home Messages
> Clinicians need to consider that most critically ill patients have micronutrient deficiencies.
> Factors influencing micronutrient deficiencies in critical illness include insufficient intake, increased losses, and drug effects.
> Individualized monitoring of micronutrients is necessary: early and continuous monitoring in critical illness.
> Daily nutritional therapy for critically ill patients should provide the basic intake of micronutrients.
> For high-risk ICU patients, consider additional supplementation of Vitamin C, Vitamin D, B12, folate, zinc, and carnitine.
References
[1] ESPEN micronutrient guideline. [J].Clin Nutr. 2022 Jun;41(6):1357-1424.
[2] Clin Nutr. 2021 Feb;40(2):590-599.
[3] Intensive Care Med (2019)45:1136-1139.
[4] Curr Opin Crit Care 2023, 29:101-107.
[5] Clin Nutr. 2021 Jun;40(6):3780-3786.
[6] Crit Care. 2023 Jul 4;27(1):261.
[7] Curr Opin Crit Care, 2023, 29: 315-329.
[8] Curr Opin Crit Care (2020)26(4):355-362.
[9] Clin Chem Lab Med. 2017 Oct 26;55(11):1652-1668.
[10] Crit Care Resusc. 2018 Sep;20(3):174-179.
[11] Br J Clin Pharmacol. 2022 May;88(5):2327-2339.
[12] Clin Nutr ESPEN.2022 Jun:49:61-67.
[13] Crit Care Resusc. 2018 Sep;20(3):174-179.
[14] J Pediatr (Rio J). 2014;90(2):99-101.
[15] Chinese Medical Journal, 2022,102(29):2236-2255.
[16] Curr Opin Crit Care (2020)26(4):355-362.

Expert Introduction


