【Research Background】
Replacing graphite anodes (theoretical specific capacity of 372 mA h g–1) with lithium metal anodes (theoretical specific capacity of 3860 mA h g–1) is considered one of the most attractive methods to enhance battery energy density.
The electrolyte plays a crucial role in insulating electrons while conducting ions between electrodes, significantly influencing battery performance. With the rapid development of electrochemical energy storage technologies, traditional electrolytes can no longer meet the demands of high energy density batteries. Moreover, lithium metal as the ultimate anode material places high demands on the electrolyte. The safety hazards posed by lithium dendrite growth and flammable electrolytes are the main issues restricting the practical application of lithium metal anodes. In addition to optimizing traditional electrolytes, various electrolyte design concepts have been proposed, including super-concentrated, polymer, solid-state, ionic liquids, and eutectic electrolytes. Among these conceptual electrolytes, eutectic electrolytes are a branch of deep eutectic solvents, typically prepared from a eutectic mixture of Lewis or Brønsted acids and bases. The intermolecular interactions between different components lead to a low melting point. Eutectic electrolytes are usually composed of metal salts and hydrogen bond donors, promoting advancements in the field of metal battery applications. Eutectic electrolytes have attracted significant research interest due to their non-toxic, low-cost, and environmentally friendly special properties compared to organic carbonates and ionic liquid electrolytes (Figure 1a). However, research on eutectic electrolytes is still in its early stages. Despite the many advantages of eutectic electrolytes, high viscosity and low ionic conductivity are the main issues limiting their application in energy storage.
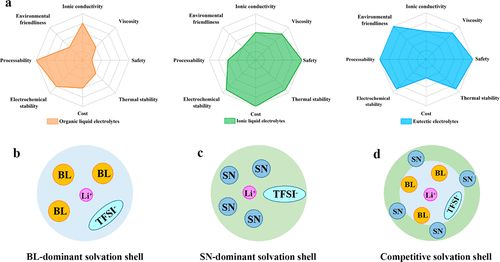
Figure 1. (a) Radar chart of different electrolyte properties, with the highest values representing the best characteristics. (b) Solvation shell dominated by BL, (c) solvation shell dominated by SN, and (d) schematic diagram of competitive solvation shell.
【Work Overview】
Recently, Professors Ci Lijie, Li Mingyu, and Zhang Jiaheng from Harbin Institute of Technology (Shenzhen) and their team designed a ternary eutectic electrolyte composed of LiTFSI (TFSI = bis(trifluoromethanesulfonyl)imide), butyrolactam (BL), and succinonitrile (SN). This electrolyte exhibits high ionic conductivity, non-flammability, and a wide electrochemical window.
Additionally, a “competitive solvation” mechanism between SN and BL was proposed to understand the improved characteristics of the ternary eutectic electrolyte (Figure 1b-d). The competitive solvation effect between SN, BL, and Li+ reduces viscosity and enhances the stability of the eutectic electrolyte. The preferential coordination of BL to Li+ favors the formation of a stable solid electrolyte interphase (SEI) membrane, resulting in uniform and dendrite-free Li plating. Benefiting from the advantageous properties of the ternary eutectic electrolyte, LiFePO4 (LFP)||Li batteries demonstrate outstanding cycling performance and higher Coulombic efficiency, even under harsh conditions such as high current density, low temperature, and high temperature.
Using this ternary eutectic electrolyte, the LiFePO4/Li battery maintains a capacity retention of up to 90% after 500 cycles at 2C, with an average Coulombic efficiency of 99.8%. Nickel-rich LiNi0.8Co0.1Al0.1O2/Li and LiNi0.8Co0.1Mn0.1O2/Li batteries based on modified ternary eutectic electrolyte have achieved excellent cycling performance. This research provides insights for understanding and designing better electrolytes for lithium metal batteries and similar sodium/potassium metal batteries.
This result was published in the top international journal ACS Nano under the title “A Competitive Solvation of Ternary Eutectic Electrolytes Tailoring the Electrode/Electrolyte Interphase for Lithium Metal Batteries“. The first author is Wu Wanbao.
【Specific Content】
Physical and Chemical Properties of Ternary Eutectic Electrolytes
The physical and chemical properties of eutectic electrolytes are shown in Figure 2b. In binary eutectic electrolytes, LB (with a molar ratio of LITFSI to BL of 1:3) shows a conductivity of 0.43 mS cm–1 and a high viscosity of 238.1 mPa s. Compared to LB, the LS electrolyte, composed of LiTFSI and SN with a molar ratio of 1:4, exhibits a high conductivity of 1.72 mS cm–1 and a relatively low viscosity of 86.0 mPa s. For the ternary eutectic electrolyte, the viscosity decreases with increasing SN content. As the molar ratio of SN increases from 1 to 5, the viscosity decreases significantly from 137.6 mPa s to 27.5 mPa s, while the conductivity increases from 1.25 to 2.83 mS cm–1. Notably, at a molar ratio of 5 for SN, the lithium salt concentration in the electrolyte is only 1.15M, which is close to the commercial electrolyte concentration of 1M. Furthermore, LB and LiTFSI, BL, and SN (LBS) with fluoroethylene carbonate (FEC) as an additive can form a stable SEI membrane to prevent further reactions of BL with metallic lithium.
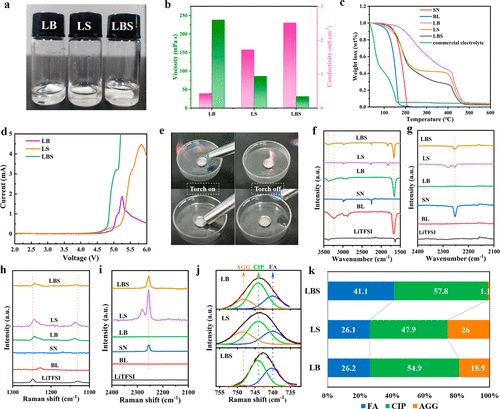
Figure 2. Comparison of properties of different electrolytes and physical-chemical properties of eutectic electrolytes.
The thermal stability of the electrolyte is of considerable importance to battery safety in cases of short circuits or thermal runaway. As shown in Figure 2c, pure BL loses 50.45% of its weight at 150°C. However, only a weight loss of 3.06% is observed in LB, attributed to the strong interactions between LiTFSI and BL after forming the eutectic electrolyte, which suppresses the decomposition of BL. Pure SN loses only 12.09% at 150°C; hence, LS has good thermal stability, losing 13.73%. For the ternary eutectic electrolyte, all electrolytes exhibit approximately 10% weight loss at 150°C. In contrast, commercial carbonate electrolytes show a weight loss of 86.26% at 150°C. The good thermal stability of the ternary eutectic electrolyte can be attributed to the interactions between LiTFSI, BL, and SN, as well as the inherent thermal stability of SN. When the molar ratio of SN is 4, the lowest eutectic temperature of the ternary eutectic electrolyte is observed at -86.95°C, which increases to -73.14°C when the molar ratio of SN is reduced to 1. The eutectic temperature of the ternary eutectic electrolyte is much lower than that of the binary eutectic electrolyte LB by 1.7°C. Notably, when the molar ratio of SN increases to 5, two crystallization peaks appear at -46.53 and -84.43°C, possibly due to the phase transition of SN. The electrochemical stability of binary and ternary eutectic electrolytes was tested using LSV.
As shown in Figure 2d, the results indicate that the oxidation resistance of these electrolytes exceeds 4.5V. This suggests compatibility with most lithium battery systems, including high-voltage cathode materials. Meanwhile, aluminum current collectors can be passivated in ternary eutectic electrolytes. Considering the physical and chemical properties and cost factors, the ternary eutectic electrolyte with a molar ratio of SN set to 4 was selected as a representative, defined as LBS. For comparison, binary eutectic electrolytes LB and LS as well as a commercial electrolyte were also studied.
Flammability tests were conducted on commercial electrolytes and ternary eutectic electrolytes (Figure 2e). Commercial electrolytes are easily ignited, posing significant safety risks to batteries in practical applications. In contrast, ternary eutectic electrolytes do not support combustion, indicating good non-flammability and safety.
Solvation Structure Analysis of Ternary Eutectic Electrolytes
The formation mechanism of ternary eutectic electrolytes was investigated using FTIR and Raman spectroscopy. Competitive solvation exists between BL and SN with Li+, with BL preferentially forming solvation structures with Li+, as shown in Figure 1d. Additionally, Figure 1b shows the solvation structure of LB without SN, indicating that BL participates in the first solvation shell. The bonds of the original components are collectively weakened by the intermolecular interactions between LiTFSI, BL, and SN, leading to the eutectic solution. (Figure 2a). The competitive solvation in ternary eutectic electrolytes results in low viscosity, high ionic conductivity, and stable electrolytes.
Morphological Analysis of Lithium Deposition and Theoretical Simulation of Competitive Solvation
The compatibility of different electrolytes with lithium metal anodes was evaluated through cycling Li||Li symmetric cells (Figure 3a). The ternary eutectic electrolyte with competitive solvation can effectively regulate morphology, achieving dendrite-free lithium deposition. Compared to binary eutectic electrolytes, using ternary eutectic electrolytes results in more stable cycling performance of lithium-ion symmetric batteries.
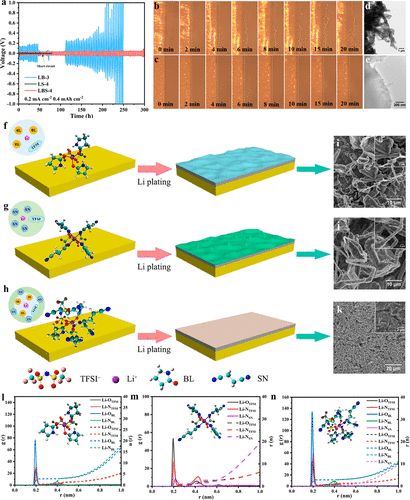
Figure 3. Electrochemical symmetric battery tests of different eutectic electrolytes, morphology of lithium deposition, and theoretical calculations.
Theoretical simulations can better understand the competitive solvation mechanism and ionic specifications of ternary eutectic electrolytes. According to Figure 3l-n, the radial distribution functions and coordination numbers of LB, LS, and LBS indicate that the first Li+ coordination shell in LB (within 2.80 Å) is primarily dominated by Li-OBL and Li-OTFSI at 1.93 Å, with coordination numbers of 2.85 and 1.08, respectively. For LS, peaks in the radial distribution functions (RDF) at 1.93 Å and 2.16 Å are clearly observed, attributed to Li-OTFSI and Li-NSN. This indicates that SN molecules participate in the solvation structure of Li+. By adding SN to form the ternary eutectic electrolyte (LBS), the RDF peak corresponding to the Li-OBL coordination structure at 1.93 Å significantly increases with the increasing SN content. This suggests an enhancement in coordination between Li+ and BL. (31)For LB and LS, each Li+-solvation shell is often coordinated with two or three TFSI– anions. In contrast, in the case of LBS, only one TFSI– anion (on average) is observed in the Li+-solvation shell (Moreover, the coordination number of Li-OTFSI decreases from 1.08 in LB to 0.93 in LBS, indicating that the introduction of SN facilitates the dissociation of Li+ and TFSI–. Notably, no RDF peak for Li-NSN is observed in the ternary eutectic electrolyte. Furthermore, the coordination number of Li-NSN significantly decreases from 2.11 in LS to 0.04 in LBS, indicating that SN does not participate in the solvation shell. These MD results corroborate the FTIR and Raman characterization results, further confirming the existence of competitive solvation structures in ternary eutectic electrolytes.
In the case of LB, Li+ coordinates with the C═O of BL and the S═O of TFSI– anions, and as the amount of BL increases, Li+ preferentially coordinates with BL, facilitating the dissociation of Li+ and TFSI– anions. For the LS binary system, Li+ and SN are observed to form Li-N (approximately 2 Å), even when the amount of SN increases to 4. This is consistent with the observation of solvation structures involving LiTFSI and SN in LS using Raman spectroscopy. Additionally, the coordination of LiTFSI with different amounts of BL and SN in ternary eutectic electrolytes was verified, where Li+ preferentially coordinates with BL in the presence of SN, especially when the amount of BL exceeds 3. This verifies that SN does not participate in the solvation structure. Reduced Density Gradient (RDG) analysis shows weak intermolecular interactions between LiTFSI, BL, and SN. ESP analysis determined the electronic density of LBS, indicating that the negative electronegative regions of O atoms in BL and N atoms in SN suggest a trend of coordination with the positive charge regions around Li+, leading to a stable eutectic structure. The LiTFSI(BL)3 (SN)2 structure exhibits the lowest energy and a more uniform distribution of molecular electrostatic potential energy surfaces, indicating a high tendency for this coordination structure. This competitive solvation structure plays a crucial role in the formation of solid electrolyte interphase (SEI) membranes and electrolyte stability. Species coordinated with Li+ ions are strongly polarized and preferentially reduced, forming the main components of the SEI membrane. Therefore, in ternary eutectic electrolytes, the strong interactions between BL and TFSI– with Li+ largely determine the composition and structure of the SEI membrane. In contrast, SN does not participate in the solvation structure, reducing spontaneous chemical reactions between SN and metallic Li. Thus, the addition of SN reduces the viscosity of the electrolyte and enhances ionic conductivity.
Electrochemical Performance of Batteries

Figure 4. Electrochemical performance of LFP/Li batteries using different electrolytes: (a) Rate performance of LFP/Li batteries using LBS and commercial electrolytes at 25°C. Cycling performance and corresponding Coulombic efficiency of LFP/Li batteries using different electrolytes at 25° (b) at 1C and (c) at 2C, and (d) at 5C. (e) Low-temperature performance of LFP/Li batteries using LBS at 0C and 0.1C. (g) Cycling performance and corresponding Coulombic efficiency of high-load LFP/thin Li full batteries using LBS electrolyte at 0.5C, 25°C.

Figure 5. Electrochemical performance of NCA/Li batteries using different electrolytes. (a) Rate performance of NCA/Li batteries using different electrolytes at 25°C. (b) Cycling performance and corresponding Coulombic efficiency of NCA/Li batteries at 1°C using different electrolytes. (c) Cycling performance of NCM811/Li batteries at 0.2°C. Electrochemical impedance spectroscopy results of NCA/Li batteries using (d) LBS and (e) LBS-M during different cycles.
Interface Analysis Between Electrode and Electrolyte
X-ray photoelectron spectroscopy (XPS) analysis was performed on the NCA cathode during cycling to understand why LBS-M electrolyte exhibits better cycling stability than LBS electrolyte. As shown in Figure 6a, the presence of LiF in the F 1s spectrum indicates the decomposition of LiTFSI at high charge voltages. Additionally, the presence of B-F and B-O in LBS-M indicates that LiDFOB participates in the formation of the CEI layer (Figure 6c). Specifically, the DFOB– anion gains electrons during CEI formation and is reduced to B-O bonds and oxalate rings, generating oxygen and -BF2 free radicals, forming a CEI membrane rich in B-O and B-F. The presence of F– and B– species can facilitate the formation of a dense and thin CEI layer, suppressing the dissolution of transition metal ions and reducing side reactions of the electrolyte.For the O 1s spectrum in Figure 6b, no signals of metal oxide bonds (M-O) were observed at approximately 530 eV in both electrolytes, indicating that ternary eutectic electrolytes can effectively passivate the surfaces of highly active cathodes. Effective protection of the NCA cathode by the LiDFOB additive was confirmed by TEM. After cycling both ternary eutectic electrolytes for 10 cycles, CEI layers of varying thicknesses were observed. The thickness of the LBS electrolyte was approximately 86 nanometers (Figure 6d), while that of the LBS-M electrolyte was approximately 32 nanometers (Figure 6e). The results indicate that the modified ternary eutectic electrolyte effectively protects the NCA cathode, thereby reducing further decomposition of the electrolyte during cycling.
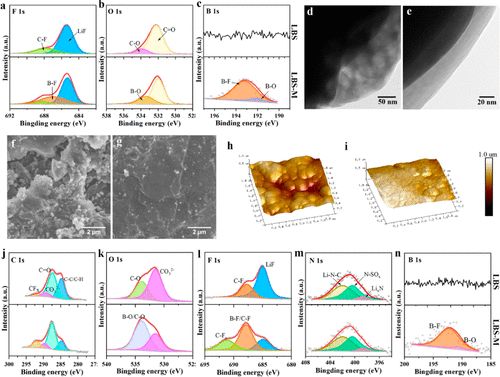
Figure 6. Characteristics of the electrode/electrolyte interface under different electrolytes. XPS characterization of CEI components on NCA cathodes cycled in LBS (top) and LBS-M (bottom).
The cycling stability of batteries is determined not only by the electrolyte’s stability on the cathode surface but also by that on the lithium metal anode. Morphological characterization of metallic lithium anodes after 100 cycles in two ternary eutectic electrolytes was performed using SEM (Figure 6f,g). The anode surface in the LBS electrolyte is loose and porous, directly exposing bulk lithium to the liquid electrolyte, leading to continuous corrosion of the lithium metal anode. The morphology of the NCA cathode surface and separator after 100 cycles also confirms that serious side reactions occur in the NCA/Li battery using LBS electrolyte. These results explain the rapid decay of battery capacity in LBS, as shown in Figure 5b. In contrast, a smooth, flat, and uniform lithium metal anode surface is clearly observed in LBS-M (Figure 6g). This indicates that the surface layer can effectively protect the metallic lithium anode from continuous corrosion by the electrolyte. These results are consistent with the atomic force microscopy (AFM) images in Figure 6h,i, confirming that the surface of lithium metal cycled in LBS-M is smoother than that cycled in LBS.
A stable SEI membrane is crucial for excellent cycling performance, which was studied using XPS spectra of cycled lithium anodes (Figure 6j-n). In the F 1s spectrum, the presence of LiF (685 eV) in both electrolytes was observed (Figure 6l), originating from the reduction of FEC or fluorinated TFSI– and DFOB– anions. Although LiF has low ionic conductivity, its low solubility in the solvent and high Young’s modulus contribute significantly to the stability of the SEI film. Peaks for B-O and B-F in LBS-M are clearly detected, as the bond energy of B-O is much weaker than that of other bonds (Figure 6n). Therefore, B-O bonds can easily break, and reactions between LiDFOB and metallic lithium participate in the formation of the SEI membrane. In the C 1s spectrum (Figure 6j), the intensity of the C-F peaks generated from the decomposition of LiTFSI in LBS-M is stronger than that in LBS, indicating more LiTFSI decomposition occurs in the presence of LiDFOB, forming the SEI membrane. Notably, high ionic conductivity species LixN appear in both ternary eutectic electrolytes, which may promote the rapid migration of Li+ and lead to uniform lithium deposition (Figure 6m). The improved ternary eutectic electrolyte not only plays a key role in regulating SEI and CEI components but also enhances compatibility with lithium metal anodes (Figure 7). The stable SEI membrane and good compatibility with metallic lithium contribute to its excellent performance.
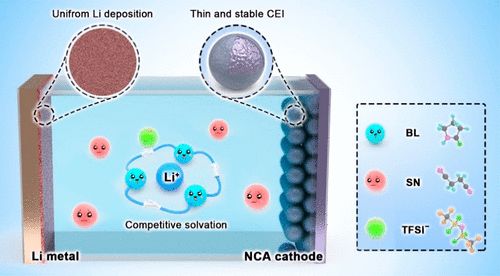
Figure 7. Schematic diagram of competitive solvation and regulation of SEI and CEI in ternary eutectic electrolytes.
【Summary】
A non-flammable ternary eutectic electrolyte has been developed, improving the cycling performance and safety of lithium metal batteries through competitive solvation structures with lithium ions. The ternary eutectic electrolyte significantly enhances the performance of LMB in three aspects.
(1) The competitive solvation mechanism reduces side reactions between SN and metallic lithium and the viscosity of the electrolyte while enhancing electrolyte stability.
(2) A stable SEI membrane with LiF and nitrogen-rich species leads to uniform and dense lithium deposition.
(3) The modified ternary eutectic electrolyte effectively improves the cathode-electrolyte interface.
Thanks to these advantages of the ternary eutectic electrolyte, both LFP/Li and NCA/Li batteries exhibit significantly extended cycle life and improved Coulombic efficiency. This research delves into the competitive solvation of electrolytes. This strategy holds promise for developing highly safe and durable lithium metal batteries, as it can benefit from the formation of ternary eutectic electrolytes with reduced lithium salt concentration and viscosity.
A Competitive Solvation of Ternary Eutectic Electrolytes Tailoring the Electrode/Electrolyte Interphase for Lithium Metal BatteriesACS Nano ( IF 18.027 ) Pub Date : 2022-08-30 , DOI: 10.1021/acsnano.2c05016Wanbao Wu, Yihong Liang, Deping Li, Yiyang Bo , Dong Wu, Lijie Ci, Mingyu Li, Jiaheng Zhang
