iNature
Activating the STING pathway in the cytosol of tumor-infiltrating antigen-presenting cells (APCs) can trigger an effective antitumor immune response for cancer treatment. However, most STING agonists are hydrophilic small molecules that face rapid clearance and poor cytosolic delivery issues after systemic administration. Although various nanoparticles have been developed to facilitate cytosolic delivery, they often suffer from premature drug release or poor cytosolic delivery during circulation.
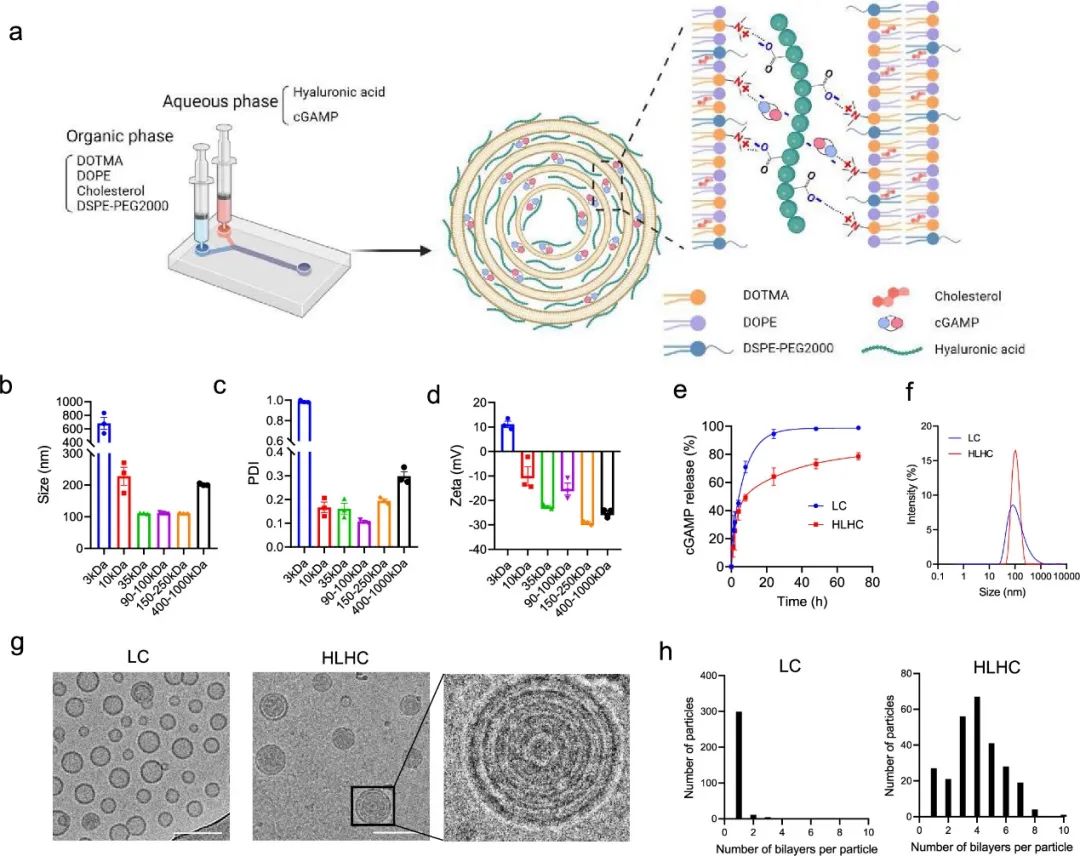
On October 17, 2024, a study titled “Single-Dose Physically Cross-Linked Hyaluronic Acid and Lipid Hybrid Nanoparticles Containing Cyclic Guanosine Monophosphate–Adenosine Monophosphate Eliminate Established Tumors” was published online by the team led by Kai Rui from Tsinghua University in ACS Nano. This study developed hybrid nanoparticles made of physically cross-linked hyaluronic acid (HA) and lipids, loaded with cyclic guanosine monophosphate-adenosine (cGAMP) (referred to as HLHC). Due to the physical cross-linking of multiple lipid layers by HA, the HLH delivery system can continuously release the drug.
HLHC effectively delivers cGAMP to the cytosol of APCs, producing more IFNβ than cGAMP and liposomal cGAMP. Compared to liposomal formulations and free drugs, HLH also improves drug circulation time and biodistribution within tumors. Notably, a single dose of HLHC, rather than liposomal cGAMP or free cGAMP, can trigger effective antitumor immunity and eliminate MC38 tumor tissue. When used in combination with αPD-L1, a single dose of HLHC effectively eliminated B16F10 tumor tissue, preventing tumor recurrence. HLHC is an effective delivery vehicle for STING agonists and can be widely used to deliver drugs that act in the cytosol.


—END—
The content is original from 【iNature】,
Please indicate the source as 【iNature】
Add WeChat Group
iNature gathers 40,000 life science researchers and doctors. We have formed 80 comprehensive groups (16 PI groups and 64 doctoral groups), and have also established specialized groups in related fields (plants, immunology, cells, microbiology, gene editing, neuroscience, chemistry, physics, cardiovascular, oncology, etc.).Note: Please indicate when joining the group (format: school + major + name; if you are a PI/professor, pleaseindicate as PI/professor; otherwise, it will be assumed you are a doctoral student, thank you).You can first add the editor’s WeChat ID (love_iNature), or long-press the QR code to add the editor, and then join the relevant group. Serious inquiries only.


Submission, collaboration, and reprint authorization matters
Please contact WeChat ID:13701829856 or email:[email protected]
If you find this article interesting, please click here!