
Neurology Medical Network Chinese Stroke Journal Authors: Wang Yiqing, Liu Pingguo, Shen Jiahui, Cai Zenglin
Recommended: Types of Infarction in Perforating Artery Disease and Advances in Neuroimaging Research, bring your small stool, let’s learn together…
Perforating arteries typically arise from large arteries, sending small arteries that penetrate into the brain parenchyma. These include two main types of branches: superficial perforators (or meningeal branches) and deep perforators. The former supplies the peripheral regions of the cerebral hemispheres and cerebellar hemispheres, as well as parts of the diencephalon and superficial parts of the brainstem, while the latter supplies the intermediate regions of the cerebral hemispheres, the apex of the cerebellum, parts of the diencephalon, and the intermediate part of the brainstem. Obstruction of perforating arteries can lead to ischemic lesions in the deep brain, but the direct relationship between the areas supplied by perforating arteries and the areas of infarction remains unclear. Therefore, understanding the structure of perforating arteries is crucial for diagnosing deep stroke syndromes and understanding the types of infarction associated with perforating artery disease. In recent years, new imaging techniques such as high-resolution, high-field MRI have facilitated in-depth studies of the anatomical structure of perforating arteries and the different types of infarction associated with them. This article primarily reviews the perforating arteries of the anterior and posterior circulation, such as the Heubner artery, lenticulostriate artery, anterior choroidal artery (AChA), and medullary arteries, the relationship between their blood supply areas and adjacent vessels, the types of infarction associated with perforating artery disease, and their imaging advancements.
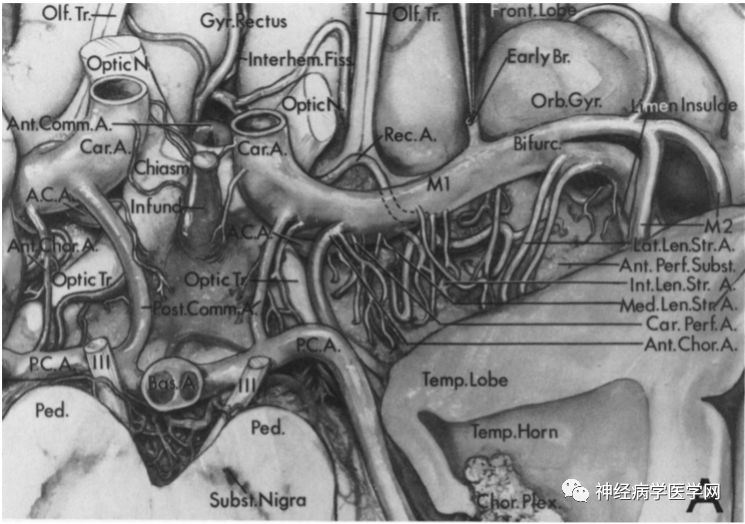
1. Different Blood Supply Areas and Types of Infarction in Perforating Arteries
1.1 Blood Supply Areas and Types of Infarction in Superficial Perforators
The superficial branches of the middle cerebral artery descend towards the upper part of the lateral ventricle and supply the centrum semiovale. The centrum semiovale is a semi-oval shaped white matter area at the center of the cerebral hemisphere, mainly composed of the radiating fibers of the corpus callosum and the projection fibers passing through the internal capsule [5-6]. Most scholars define infarction in the superficial perforating artery area (superficial perforator infarction, SPI) as infarction occurring within the cortical band and outside the deep perforator level of the corona radiata. Unlike infarction located in the internal border-zone area adjacent to the lateral ventricle (internal border-zone infarction, IBI), SPI is often located laterally and is widely dispersed [1] (as shown in Figures 1 and 2).
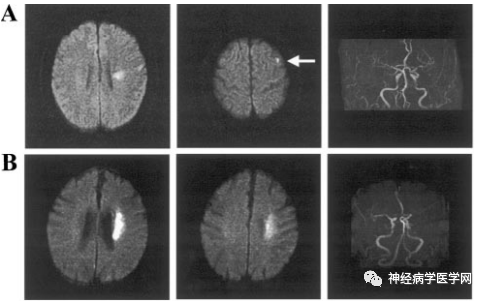
Figure 1 A: Characteristics of DWI infarction in a 72-year-old male patient with SPI and MRA findings. B: Characteristics of DWI infarction in a 64-year-old male patient with IBI.
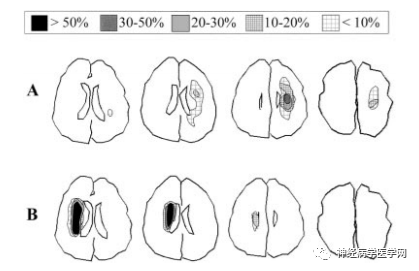
Figure 2 Diagram illustrating the distribution characteristics of infarction in SPI (A) and IBI (B) on DWI.
The pathogenesis of SPI remains controversial. Domestic scholars Wu Fang et al. [7] assessed the characteristics of intracranial large artery wall and lumen stenosis in patients with SPI and deep perforating artery area infarction (deep perforator infarction, DPI) using HR-MRI, confirming that the incidence of atherosclerotic plaques in intracranial large arteries in SPI patients is higher than that in DPI patients, and the degree of lumen stenosis in SPI patients is greater than that in DPI patients (as shown in Figures 3, 4, and 5).
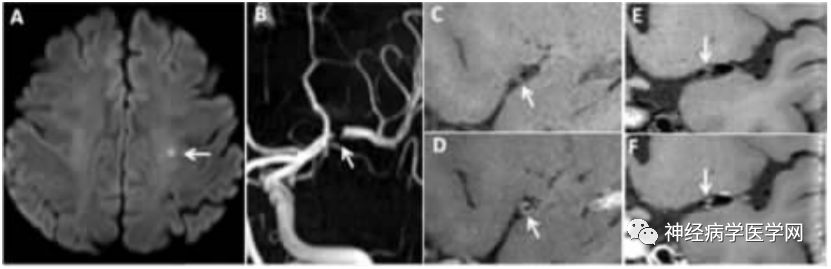
Figure 3: SPI Patient. A DWI shows punctate high signal in the left centrum semiovale. B TOF-MRA shows interruption of blood flow signal in the left middle cerebral artery (MCA) M1 segment. C, D Sagittal HR-MRI images before (C) and after (D) enhancement show eccentric plaque with enhancement (grade 1) in the left MCA, with a degree of lumen stenosis of 66.7%. E, F Coronal HR-MRI images before and after enhancement show isolated plaque in the left MCA trunk.
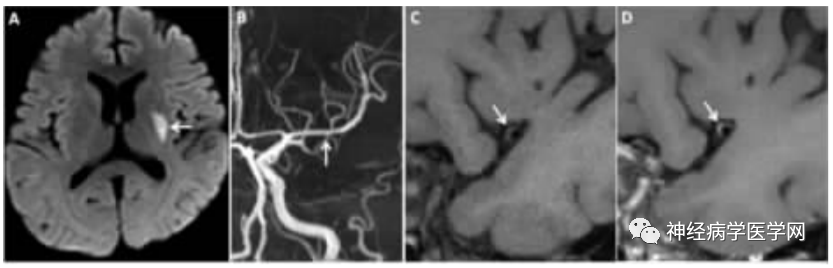
Figure 4: DPI Patient. A DWI shows high signal lesions in the left basal ganglia area. B TOF-MRA shows mild irregularity in the lumen of the left MCA-M1 segment. C, D Cross-sectional HR-MRI images, before (C) and after (D) enhancement show eccentric plaque with enhancement (grade 1) in the left MCA, with a degree of lumen stenosis of 33.3%.
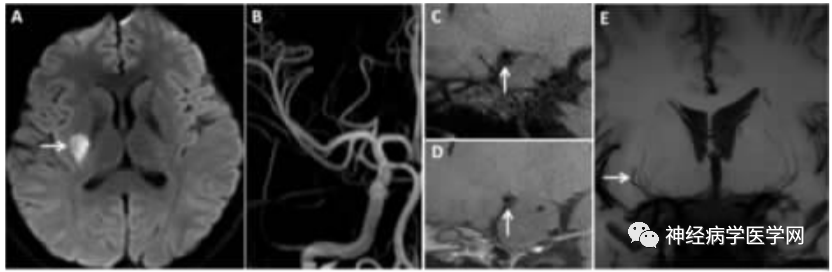
Figure 5: DPI Patient. A DWI shows high signal lesions in the right basal ganglia area. B TOF-MRA shows no clear stenosis in the right intracranial large arteries. C, D Sagittal HR-MRI images, before (C) and after (D) enhancement show no abnormal thickening or enhancement of the right MCA wall. E Coronal minimum density projection image shows the right MCA and its perforating artery are shorter than the contralateral side.
1.2 Blood Supply Areas and Types of Infarction in the Heubner Artery
The Heubner artery branches off near the anterior communicating artery from the anterior cerebral artery (at the A1–A2 junction), and can have 1-3 perforating branches, entering the anterior part of the anterior limb of the internal capsule, the anterior third of the putamen, the anteromedial part of the globus pallidus, the knee/ventral part of the internal capsule, the anterior part of the anterior commissure, and the anterior nucleus of the hypothalamus, mainly supplying the anterior ventral part of the basal ganglia, frontal lobe, olfactory bulb, and anterior part of the hypothalamus, as well as the optic nerve [9].
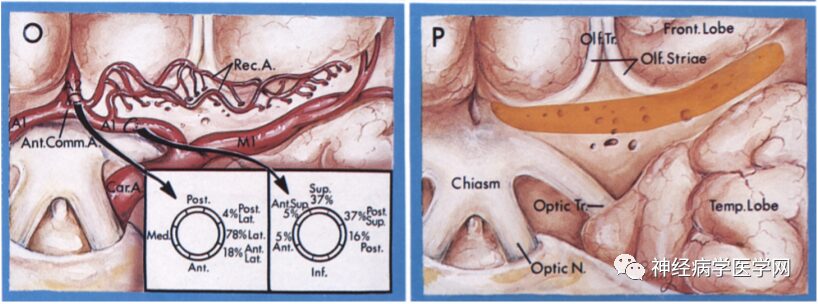
Figure 6 O: The Heubner artery branches off near the anterior communicating artery from the anterior cerebral artery (at the A1–A2 junction); P: Entering the brain parenchyma from the anterior limb.
Some scholars believe that the blood supply area of the Heubner artery corresponds to the marginal zone, while the blood supply area of the lateral lenticulostriate artery corresponds to the sensory and motor areas, and the blood supply area of the medial lenticulostriate artery corresponds to the lateral marginal zone and the ventromedial sensory and motor areas [10]. Unilateral damage to the Heubner artery can lead to contralateral arm paralysis, contralateral facial paralysis, dysarthria, and hemiballismus, while bilateral damage can lead to akinetic mutism. Although the Heubner artery and the lenticulostriate artery are terminal arteries from different large arteries, they supply the same area of the basal ganglia, and their types of infarction can be divided into two patterns: corona radiata and centrum semiovale [11] (as shown in Figure 6).
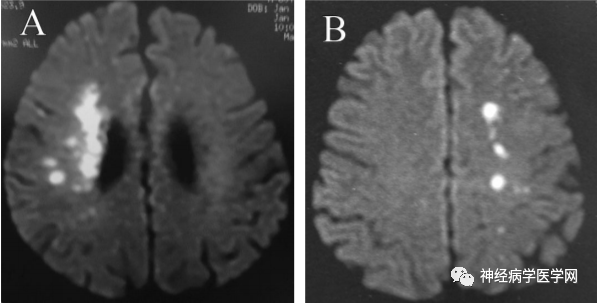
Figure 7: Two patterns of corona radiata (A) and centrum semiovale (B).
1.3 Blood Supply Areas and Types of Infarction in the Lenticulostriate Artery
The lenticulostriate artery is a perforating branch that arises from the horizontal portion of the middle cerebral artery trunk (M1 segment), consisting of 2-12 branches (averaging 7.1 branches), and sometimes has an additional branch in the M2 segment. The lenticulostriate artery branches off from the proximal to distal part of the M1 segment in an onion-skin-like arrangement, with the branches originating from the proximal part being thinner and those from the distal part being thicker, with branch diameters generally between 700-800μm, after which the vessel diameter expands [12]. According to the origin points from the M1 segment, the lenticulostriate artery can be divided into three groups: medial, intermediate, and lateral groups [13]. Marinković et al. [14] found that the medial group supplies the interior of the putamen and the dorsal side of the globus pallidus, while the intermediate and lateral groups form the external group, supplying most of the external putamen, the external globus pallidus, above the caudate nucleus, and the internal capsule [9] (as shown in the figure).
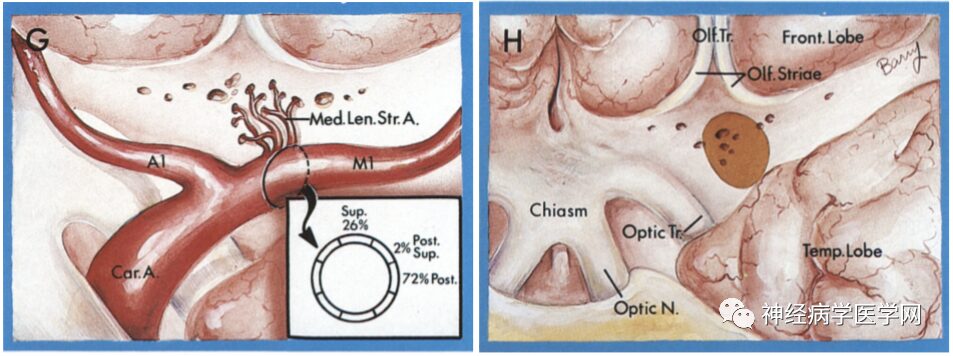
Figure 8 G: The medial group of the lenticulostriate artery branches off from the proximal part of the M1 segment; H: Enters the brain parenchyma from the medial part of the anterior limb.

Figure 9 I: The intermediate group of the lenticulostriate artery branches off from the M1 segment, with complex terminal branching that resembles a branched candelabrum entering the anterior limb; J: Enters the brain parenchyma from the medial part of the anterior limb.
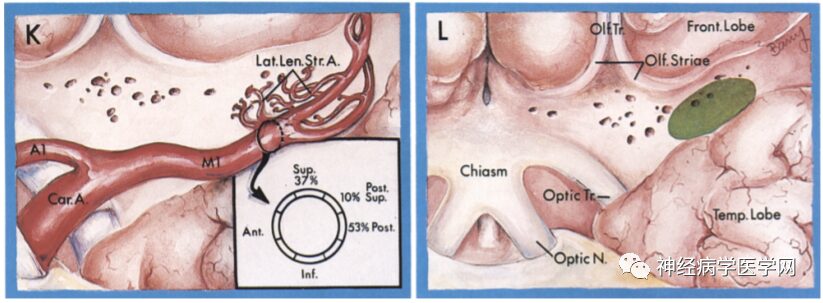
Figure 10 K: The lateral group of the lenticulostriate artery originates at the bifurcation of the middle cerebral artery, branching near M1 and M2, and extends in an S-shaped pattern; L: Enters the brain parenchyma from the medial part of the anterior limb.
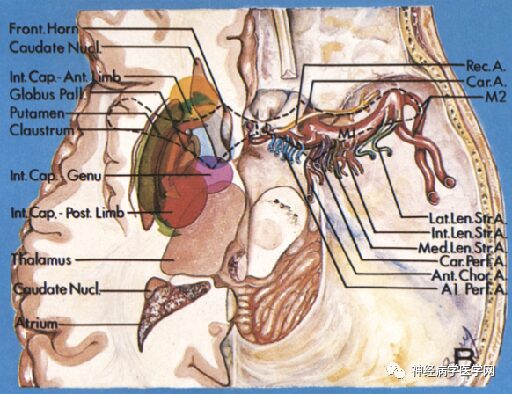
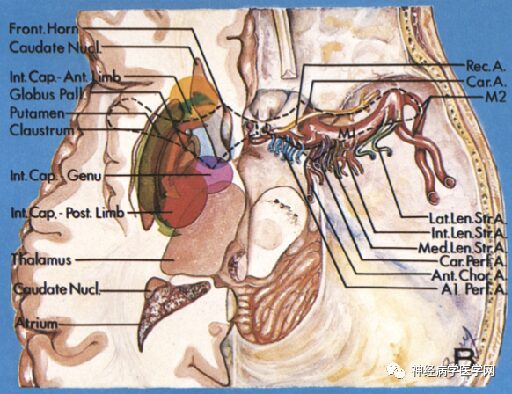
Figure 11: Distribution map of various perforating arteries supplying through the anterior limb.
The lenticulostriate artery branches off at right angles and extends in the opposite direction to the flow direction of the M1 segment (i.e., inward), thus experiencing greater blood flow impact. The types of infarction in the blood supply area of the lenticulostriate artery include lacunar infarction, branch atheromatous disease (BAD) type infarction, and striatocapsular infarction (SCI). In 1989, Caplan [15] first proposed the concept of BAD to describe stenosis or occlusion at the origin of deep perforating arteries, which is related to small artery atherosclerosis or plaque at the origin and can lead to small infarcts in areas such as the internal capsule or pons. Fisher [4] noted that lipohyalinosis occurs in vessels with a diameter of 200μm or smaller, leading to lacunar infarction 2-5mm distal to the branches of the lenticulostriate artery. If the origin of the perforating artery is blocked due to microatherosclerosis, it can cause complete infarction of the entire perforating artery, which is considered BAD type infarction [15-16]. However, even if not originating from the lenticulostriate artery, most lenticulostriate artery infarctions are based on microatherosclerosis in vessels measuring 300-700μm in diameter. Therefore, in many lacunar infarction cases, the pathological basis of microatherosclerosis is more common than lipohyalinosis [17]. If the origin of the perforating artery is occluded, the BAD type infarction of the lenticulostriate artery can be divided into anterior/posterior types: the posterior type damages the corticospinal tract, which can often predict that the patient will experience progressive motor deficits and severe paralysis [18] (as shown in the figure).
The anterior/posterior classification method for BAD type infarction is as follows:
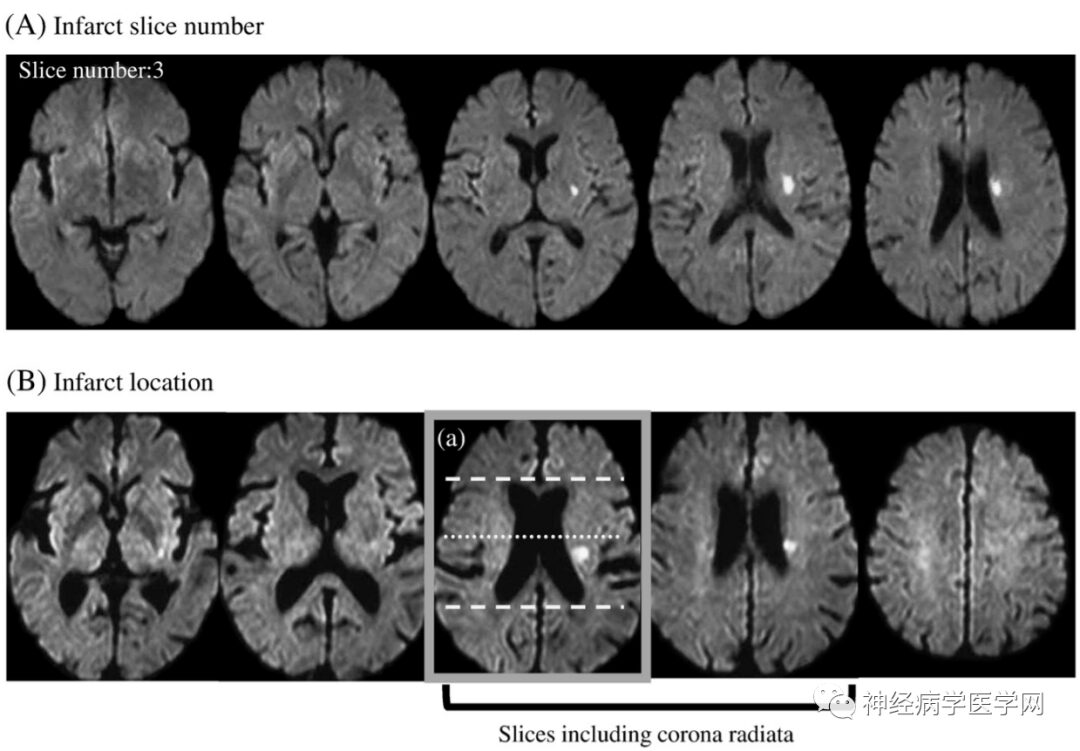
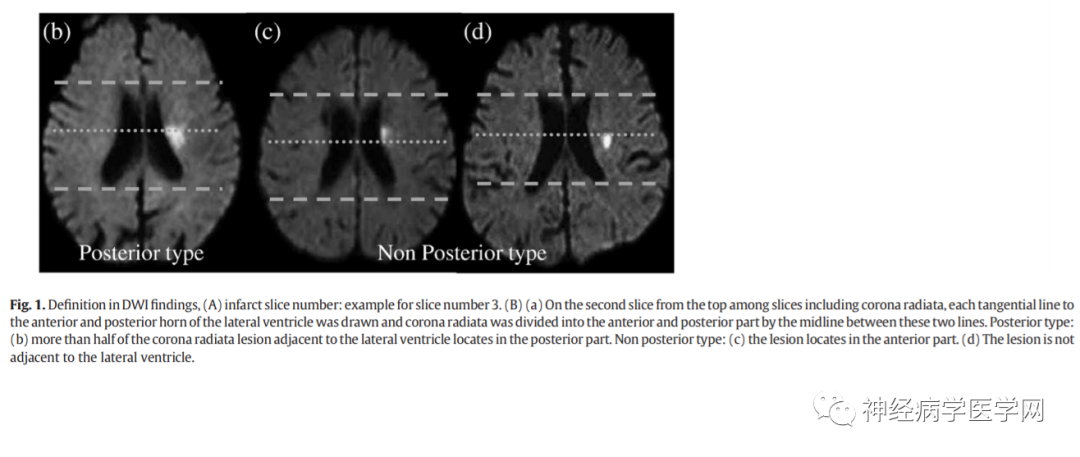
Another example is shown in Figure 12:
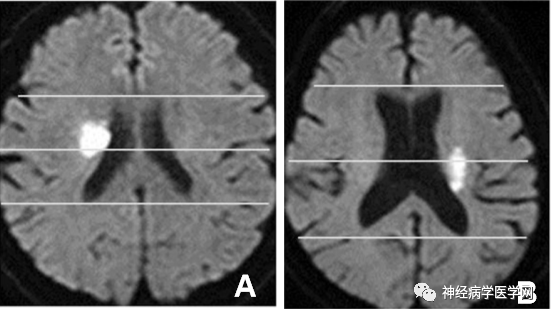
Figure 12: The anterior and posterior types of BAD type brain infarction divided by the midpoint horizontal line of the anterior-posterior diameter of the lateral ventricle.
The clinical significance of this classification is that posterior type infarction can often predict that the patient will experience progressive motor deficits, as the posterior type damages the corticospinal tract.
Striatocapsular infarction (SCI) is a relatively special type of basal ganglia infarction. In 1984, Bladin et al. [20] proposed the concept of SCI, defining a diameter of ≥3cm as the “gold standard” for diagnosing SCI, and believed that the infarct must include at least part of the head of the caudate nucleus and the internal capsule or the putamen and the internal capsule, which can be associated with various cortical symptoms. SCI typically manifests as multiple lenticulostriate artery infarcts with diameters of 2.0-3.0cm, presenting in forms such as lens-shaped, comma-shaped, and triangular infarcts [17]. The most common cause of SCI formation is embolism, but it can also be based on atherosclerosis. Total infarction of the striatocapsular region can observe two patterns centered on the putamen and the head of the caudate nucleus, respectively. Additionally, depending on the extent of ischemia, there can be a “mottled” ischemia of single vessel infarction rather than uniform infarction lesions.
1.4 Blood Supply Areas and Types of Infarction in the Anterior Choroidal Artery (AChA)
After the internal carotid artery gives off the ophthalmic artery and posterior communicating artery, the AChA branches off laterally, traveling posteriorly and laterally along the optic tract, penetrating into the globus pallidus and the posterior limb of the internal capsule, then sending branches responsible for the blood supply of parts of the temporal lobe and some parts of the midbrain and thalamus, ultimately terminating at the lateral geniculate body and the choroid plexus at the inferior angle of the lateral ventricle. The part of the AChA before it enters the choroidal fissure is referred to as the pool section, while the part after it enters the choroidal fissure is referred to as the ventricular section.
The pool section has three main vascular branches: ① branches distributed laterally to supply the medial temporal lobe (amygdala, uncus, anterior part of the hippocampus); ② branches extending inward to supply the cerebral peduncles and midbrain; ③ branches that penetrate upward to supply the internal capsule and basal ganglia [17, 22]. Some scholars also divide the third branch into proximal and distal branches, with the proximal branch supplying the knee of the internal capsule and the internal globus pallidus, and the distal branch supplying the posterior limb of the internal capsule and the lateral thalamus [22]. The internal capsule is the convergence point of the brain’s afferent and efferent nerves, where the pyramidal tract, thalamocortical tract, optic radiation, and auditory radiation converge. The anterior 2/3 of the posterior limb of the internal capsule is where the corticospinal tract passes through, while the posterior 1/3 is where the thalamocortical tract, optic radiation, and auditory radiation fibers pass through. Ischemic damage to the internal capsule typically results in contralateral limb paralysis, contralateral sensory loss, and homonymous hemianopia, known as AChA syndrome [9]. Due to collateral circulation, different types of AChA infarction can vary; in cases of internal carotid artery occlusion, blood flow from the posterior communicating artery and/or anterior communicating artery and the ipsilateral anterior cerebral artery serves as collateral flow for the occluded internal carotid artery. If collateral circulation is sufficient, the AChA blood supply area may be spared from infarction [23-24]. The AChA has a small diameter, and its infarction typically presents as lacunar infarction, which may be caused by obstruction of the perforating arteries, but large artery or cardiac embolism can also lead to complete infarction of the AChA blood supply area [25].
1.5 Blood Supply Areas and Types of Infarction in Medullary Branches
The centrum semiovale is the boundary area between the cortical branches of the middle cerebral artery and the anterior cerebral artery. The vessels supplying the centrum semiovale are the medullary branches, which branch vertically from the cerebral cortex into the medulla. Medullary branches are also referred to as superficial perforators, to distinguish them from deep perforators of the basal ganglia and thalamus. Infarction of the medullary branches can cause unique branch infarcts that are almost vertical to the ventricles. Yonemura et al. [26] noted that embolic lesions in medullary branch infarctions are more common than stenosis/occlusion lesions of the internal carotid artery and middle cerebral artery. Some scholars conducted transesophageal echocardiography on 79 patients with medullary infarctions and found that 45 cases (57.0%) were embolic brain infarctions, indicating that more than half were caused by patent foramen ovale, complex aortic plaque lesions, atrial fibrillation, or carotid artery lesions, while other non-embolic brain infarctions were lacunar infarctions.
Medullary branch infarction is a type of small infarction, often considered a small vessel disease; however, it is necessary to dynamically monitor for emboli, and in some cases, examinations including transesophageal echocardiography are needed to search for embolic sources. Standard imaging is often inadequate to distinguish between medullary branch infarctions and lenticulostriate artery infarctions. The long island artery, which branches from the M2 segment of the middle cerebral artery towards the anterior horn of the ventricle, is important for distinguishing between the blood supply areas of the medullary branches above the A-I line and the vascular territory of the striatal arteries below it. The centrum semiovale is the boundary area between cortical branches, and boundary zone infarction mainly occurs in cases of internal carotid artery stenosis or occlusion, where transient brain perfusion insufficiency can lead to multiple discontinuous small infarcts, with coronal sections more likely to reveal infarcts in the boundary zone between the anterior cerebral artery and middle cerebral artery [28]. Additionally, the corona radiata refers to the lower part of the centrum semiovale, which is the terminal region of the lenticulostriate artery, corresponding to the boundary area formed between the cortical branches of the middle cerebral artery and the medullary branches, referred to as internal border-zone infarction (IBZ). In cases of stenosis or occlusion of the M1 segment of the middle cerebral artery, insufficient blood perfusion in the main trunk of the middle cerebral artery leads to infarction between the lenticulostriate artery and the medullary branches, characterized by a beaded high signal distribution on DWI in the corona radiata.
2. Advances in Imaging of Perforating Artery Disease
Infarction caused by obstruction of perforating arteries is an important subtype of ischemic stroke, but for a long time, its etiological diagnosis has been challenging due to the small size of perforating arteries, making it difficult for conventional imaging methods to display. High-resolution wall imaging has high spatial resolution and can clearly show the fine structure of the walls of perforating arteries, allowing for the detection of smaller atherosclerotic plaques, thus aiding in the study of perforating artery diseases [30].
Recently developed ultra-high field 7T MRI demonstrates functionality in showing the fine structures of small perforating vessels far superior to 1.5T and 3T MRI. Additionally, the signal-to-noise ratio of 7T MRI is approximately 88% higher than that of 3T MRI. Geurts et al. applied 7T two-dimensional phase-contrast MRI (2D-PC-MRI) to measure the pulsatility index of the perforating arteries in the basal ganglia and centrum semiovale, demonstrating that patients with strokes caused by small vessel disease exhibited functional abnormalities in the perforating arteries compared to healthy controls, with fewer perforating arteries and a higher pulsatility index (PI) in patients with lacunar infarction and deep microbleeds. Arts et al. used 7T 2D-PCMRI to automatically measure blood flow velocity and pulsatility in cerebral perforating arteries, suggesting that this method can provide a more precise quantitative approach for studying microcirculation in cerebral small vessel disease, which is important for determining the correlation between perforating artery damage and vascular brain injuries (such as microbleeds, infarction, reduced integrity of deep white matter, and hemodynamic changes), and can detect early changes in patients with vascular cognitive impairment.
In recent years, many new imaging methods have been widely applied in the study of cerebral perforating arteries. Liang et al. [36] included 31 healthy subjects and 28 patients with lenticulostriate artery infarction, using 3D-TOF-MRA and three-dimensional fast spin-echo T1WI (3D-FSE T1WI, i.e., GE Healthcare’s CUBE T1) for research, showing that patients with lenticulostriate artery infarction had fewer lenticulostriate arteries on the affected side than the number of lenticulostriate arteries on the healthy side and the same side of healthy subjects. Abnormalities in the Heubner artery can lead to infarction in the centrum semiovale, and abnormalities in the lenticulostriate artery are associated with corona radiata infarction. They believe that HR 3D-FSE T1WI (CUBE T1) can visualize pathological changes in the vessel wall of perforating arteries, while HR 3D-TOF-MRA can specifically detect the number of perforating arteries, both effectively identifying perforating arteries and their types of infarction. Wang et al. used whole-brain high-resolution cardiovascular MRI (WB-HRCMR) to assess changes in lenticulostriate arteries in recent stroke patients, showing that symptomatic intracranial atherosclerotic stenosis (ICAS) lenticulostriate artery plaque characteristics were more pronounced and shorter in length compared to asymptomatic ICAS, and believed that the average length of the lenticulostriate artery is an independent predictor of stroke.
3. Conclusion
Different types of infarction occur in perforating arteries: the types of infarction in the lenticulostriate artery blood supply area include lacunar infarction, BAD type infarction, and SCI; unilateral occlusion of the Heubner artery can lead to contralateral arm paralysis, facial paralysis, dysarthria, and hemiballismus, while bilateral damage can lead to akinetic mutism; AChA lesions can cause contralateral limb paralysis, contralateral sensory loss, and homonymous hemianopia, known as AChA syndrome; infarction of the medullary branches can cause unique branch infarcts almost vertical to the ventricles and can also lead to boundary zone infarction in the centrum semiovale and corona radiata. Perforating arteries are terminal arteries, and acute ischemia can lead to lacunar infarction, while chronic ischemia can result in leukoaraiosis, which is more commonly seen clinically. Leukoaraiosis primarily occurs in the periventricular and deep white matter regions, both of which are watershed areas. Any diffuse arterial disease will exacerbate their perfusion pressure, making them susceptible to chronic ischemic damage. New neuroimaging techniques (such as 7T 2D-PC-MRI, HR 3D-TOF-MRA, HR 3D-FSE T1WI, etc.) help to determine early lesions in perforating arteries and can detect the occurrence and development of diseases at an early stage.
If you found this helpful, please click “Looking” in the lower right corner, thank you.
Follow the public account to learn which aspects of content, leave a message
