This article is reprinted from “Pharmaceutical Targeting“
Adjuvants are key components of many vaccines, enhancing the immunogenicity of antigens by improving the intensity, breadth, quality, and duration of the immune response. Since the approval of aluminum-based adjuvants by the U.S. Food and Drug Administration in 1939, the development of new adjuvants has progressed relatively slowly. Over the past century, only a few adjuvant formulations have been approved for clinical use. The application of these adjuvants must be selected based on the specific type of vaccine to elicit the desired immune response.However, the mechanisms of action for most adjuvants (including classic aluminum-based adjuvants) remain unclear, which hinders the rational design or combinatorial optimization of adjuvants to some extent.
Computer-aided drug design has been widely applied in drug discovery. Nowadays, computational methods such as pharmacophore modeling, molecular docking, and molecular dynamics (MD) simulations have also been extended to the development of innovative adjuvants, including small organic molecules, aptamers, peptides, and chemically modified nanomaterials. Macromolecular-based adjuvants are gaining increasing attention due to their advantages in modulating pharmacokinetic characteristics, enhancing binding affinity and potency with immune receptors, and promoting the internalization of antigen-presenting cells (APCs). However, the development of macromolecular-based adjuvants faces significant challenges, and reports on related computer-aided design are limited, which may be attributed to the inherent structural complexity and polydispersity of macromolecules, as well as the lack of comprehensive libraries similar to those for small molecule adjuvants.
Natural polysaccharides are the most abundant biomolecules in nature, possessing beneficial properties such as strong immunomodulatory activity, excellent biocompatibility, and low toxicity, making them attractive candidates for adjuvants. Many natural polysaccharides (such as glucans, inulin, and mannan) have been extensively studied as adjuvants. Research has shown that chemical modifications of polysaccharides can enhance their interactions with Toll-like receptors (TLRs, the most widely recognized subgroup of pathogen recognition receptors), thereby improving their adjuvant capabilities. Acetylated konjac glucomannan derivatives with an acetylation degree of 1.8 have been reported as TLR2 agonists, capable of modulating the innate and adaptive immune responses against cancer in C57BL/6 mice. Recently, acetylated chicory inulin has been identified as an effective TLR4 agonist that promotes the maturation of dendritic cells (DCs). These studies have made significant contributions, but comprehensive systematic design methods remain to be explored, limiting the chemical derivatization of parent compounds. The application of computer-aided methods in the chemical modification of polysaccharides with defined sequences is still relatively scarce.
TLRs have received increasing attention as targets for adjuvant design due to their potency in initiating signaling cascades that lead to immune activation. Among the TLR family, the membrane-bound receptor TLR4 stands out for its ability to trigger DC maturation and further elicit T helper cell and CTL responses. Recent studies have shown that TLR4 agonists can disrupt tumor-induced immune tolerance and enhance both innate and adaptive anti-cancer immune responses. Given the efficacy of TLR agonists as adjuvants for cancer vaccines and the progress of clinical trials, the computational design of novel TLR agonists for cancer immunotherapy has garnered widespread interest.
On January 9, 2025, the team of Xiao Min, Liu Changcheng, and Xu Li from the National Center for Glycoengineering Technology at Shandong University published an article titled “In Silico-Guided Discovery of Polysaccharide Derivatives as Adjuvants in Nanoparticle Vaccines for Cancer Immunotherapy” in ACS Nano (IF=16.0).

The core content is summarized in 5 points:1) Innovative Strategy
A computer-aided design method was developed to screen TLR4-targeting adjuvant candidates (Benzoylated Inulin) from 34 inulin derivatives through molecular docking and dynamics simulations.
2) Nanovaccine Construction
The selected adjuvant InBz was combined with the model antigen OVA to prepare core-shell structured nanoparticles (InBz-OVA NPs) with a particle size of approximately 100 nm, exhibiting pH-responsive release characteristics.
3) Immune Activation Mechanism
Experiments confirmed that InBz stabilizes the active conformation of the TLR4/MD2 complex (key role: locking the “molecular switch” Phe126 residue); promotes dendritic cell maturation (CD80/CD86 expression increased 2-2.5 times); enhances antigen cross-presentation (MHC I-SIINFEKL complex increased 4.6 times).
4) Significant Therapeutic Effects
In the E.G7-OVA lymphoma model: tumor volume reduced by 81%; specific CTL killing rate reached 93%; antibody titers surpassed those of the aluminum adjuvant control group; survival significantly extended (28-day survival rate 71% vs. 0% in the aluminum adjuvant group).
5) Translational Value
For the first time, a paradigm of “computational screening – chemical synthesis – immune validation” for polysaccharide adjuvant development was established, providing new tools for tumor vaccine design (proven to be extendable to other polysaccharide derivatives such as InAc/InCl).
I. Abstract
Utilizing nanoparticle (NP) structures to integrate antigens and adjuvants to enhance delivery and stimulate immune responses in cancer vaccines is becoming a promising avenue in cancer immunotherapy. However, the low immunogenicity of tumor antigens severely hinders the development of cancer vaccines. To address this challenge, this study developed a NP cancer vaccine containing polysaccharide-derived adjuvants designed through computational strategies, aiming to elicit effective antigen-specific anti-tumor immunity. Assuming TLR4 as the target receptor, the authors conducted a comprehensive evaluation of a pre-screened library containing 34 inulin derivatives through docking and molecular dynamics simulations, ultimately selecting benzoylated inulin (InBz) as the most promising TLR4 agonist. By preparing InBz NPs encapsulating the model antigen ovalbumin (OVA), the adjuvant effect was assessed. In vitro experiments indicated that InBz-OVA NPs effectively activated the TLR4 signaling pathway and promoted dendritic cell maturation, thereby enhancing antigen delivery and presentation. In vivo experiments showed that InBz-OVA NPs outperformed commercial aluminum-based adjuvants, eliciting high antibody titers, inducing antigen-specific cytotoxic T lymphocytes (CTLs), and achieving significant tumor suppression in mouse models. Furthermore, the adjuvant effects of other representative derivatives in the library (acetylated and chloroacetylated inulin) were also validated through chemical synthesis and experimental evaluation, with results consistent with computational predictions, confirming the reliability of this strategy. This study provides an effective platform for the development of efficient polysaccharide-based vaccine adjuvants.
Graphical abstract
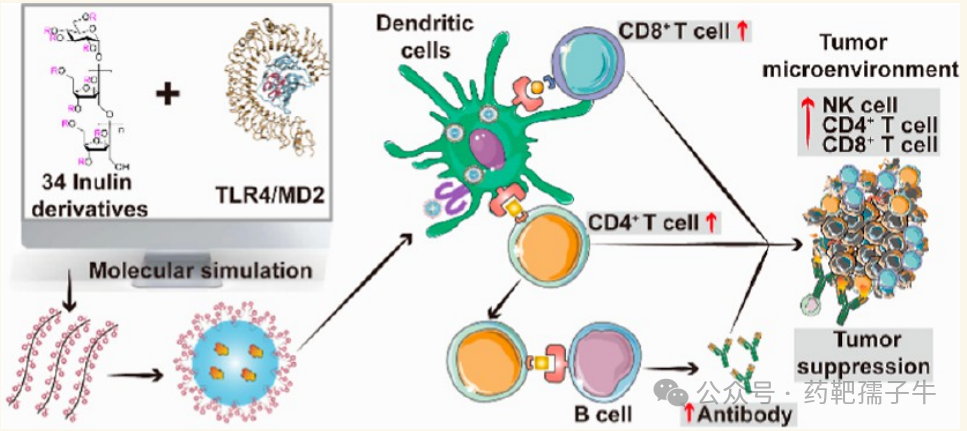
II. Research Plan
In this study, the authors developed an integrated workflow that utilizes computational methods to guide the chemical modification of polysaccharides, resulting in novel polysaccharide-based adjuvants with excellent TLR4 agonist activity to meet the needs of NP cancer vaccines. The human TLR4 complexed with myeloid differentiation factor 2 (MD2) and inulin polysaccharides was used as a model receptor-ligand pair. Based on the volume of the ligand binding pocket and the spatial dimensions of the ligand, the authors selected the reducing units of inulin (trimer, tetramer, and pentamer fragments) for their work. Subsequently, the authors constructed a library of 34 inulin derivatives, each modified with pre-screened hydrophobic chemical groups. These derivatives were ranked through flexible docking, followed by MD simulations to evaluate binding modes. Ultimately, the authors selected the optimized derivative benzoylated inulin (InBz), which exhibited the lowest binding energy and most effectively stabilized the active conformation of the TLR4/MD2 complex. Experimental validation showed that the adjuvant effect of InBz was manifested as the expression of cytokines activating the TLR4 signaling pathway in APCs, thereby stimulating robust antigen-specific T cell and B cell responses, and effectively inhibiting tumor growth in mouse models (Figure 1). The authors’ findings provide an innovative and efficient strategy for the development of potential polysaccharide-based adjuvants. Additionally, this work deepens the authors’ understanding of the molecular mechanisms underlying the immunomodulatory effects of polysaccharide derivatives, laying the foundation for the rational design of novel polysaccharide-based adjuvants.
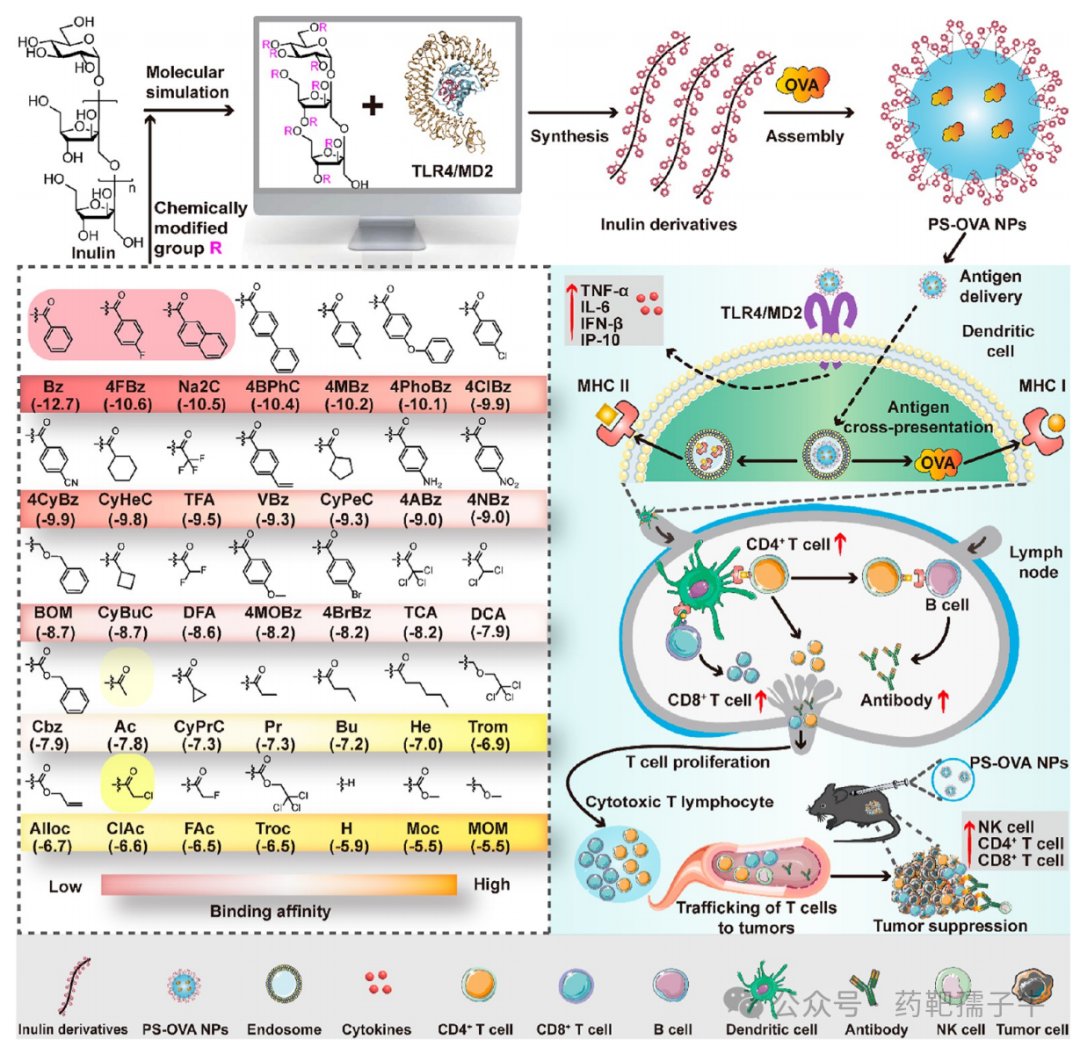
Figure 1 Schematic diagram of computer-aided design of inulin chemical modifications as adjuvants for cancer immunotherapy vaccines. Shows the chemical group structures and abbreviations used for inulin modifications (benzoyl Bz, 4-fluorobenzoyl 4FBz, etc.), with docking scores in parentheses (the lower the value, the higher the binding affinity). The shaded inulin derivatives were selected as MD simulation targets. InBz-OVA NPs promote efficient transport of antigens to lymph nodes (LNs) by activating the TLR4 signaling pathway, inducing DC maturation, and enhancing antigen presentation, ultimately eliciting a strong antigen-specific CD8+ CTL response and antibody production, leading to significant tumor regression in mouse models.
III. Results Image Display
1) Rational Design, Synthesis, and Characterization of Inulin Derivatives
See Figure1
2) Docking and Molecular Dynamics Simulation Analysis of Representative Ligands Interacting with hTLR4/MD2

Figure 2 Docking and MD simulation analysis of representative ligands interacting with hTLR4/MD2.
3) Synthesis, Characterization, and Preparation of Vaccine Delivery Nanoparticles of Inulin Derivatives
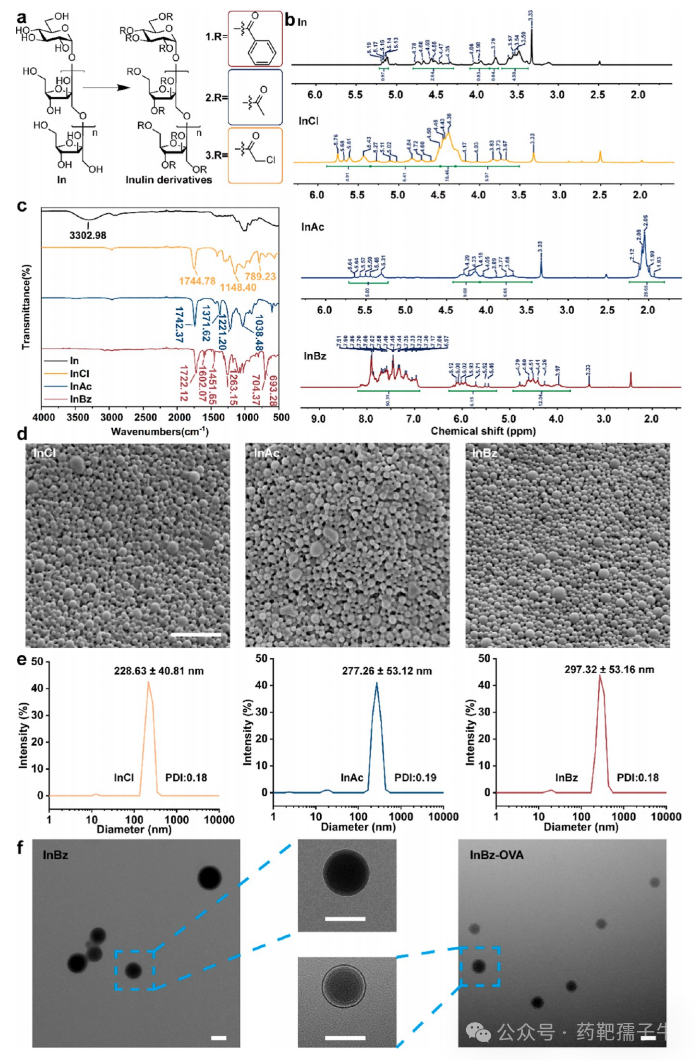
Figure 3 Synthesis characterization of inulin derivatives and preparation of vaccine delivery nanoparticles.
4) TLR4 Activation Effects and Cytotoxicity of Polysaccharide Nanoparticles (PS NPs)

Figure 4 TLR4 activation effects and cytotoxicity of PS NPs.
5) Cellular Localization of Antigen OVA+FITC and Enhanced Antigen Cross-Presentation in DC2.4 Cells
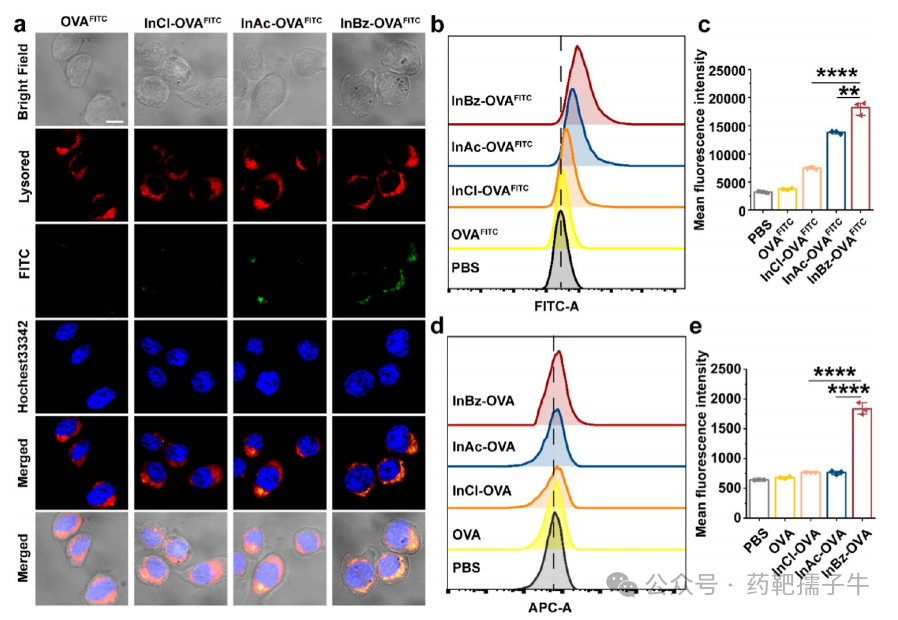
Figure 5 Cellular localization of OVA+FITC antigen and enhanced antigen cross-presentation in DC2.4 cells.
6) Enrichment of Antigen in Lymph Nodes and Activation of Antigen-Presenting Cells
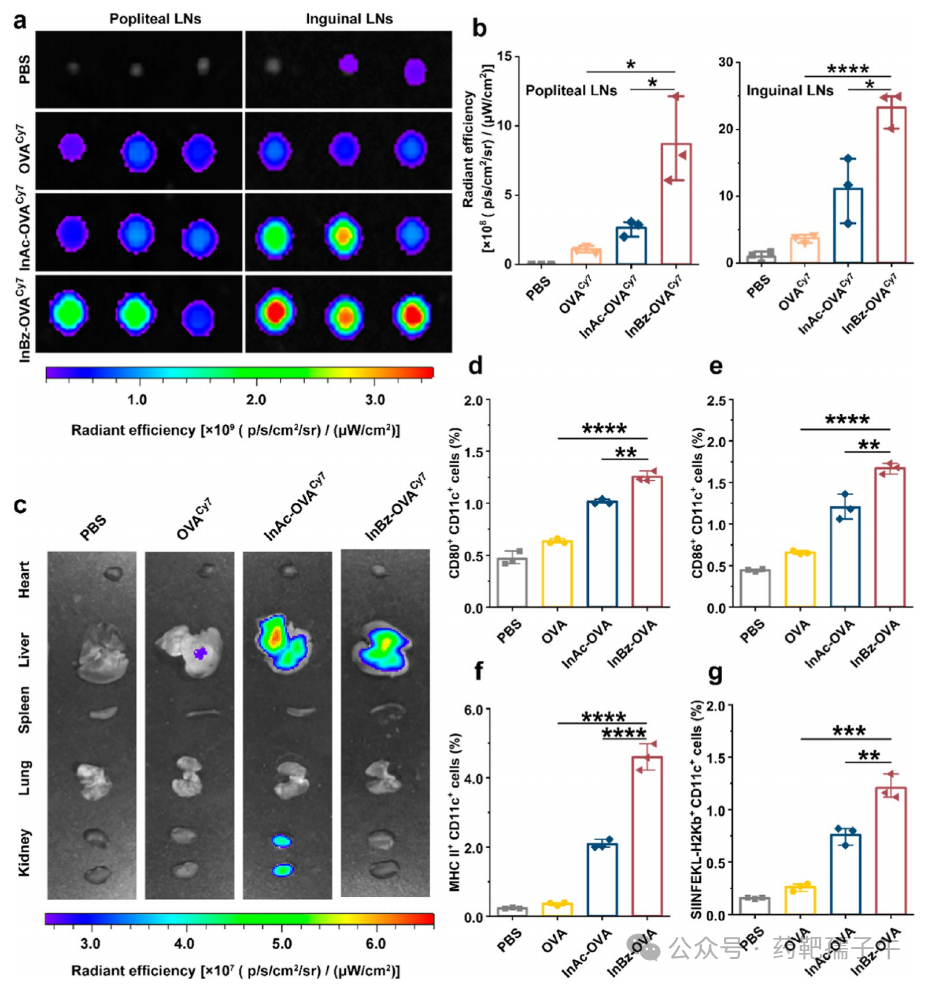
Figure 6 Enrichment of antigen in lymph nodes and activation of APCs.
7) Immune Activation Effects of PS-OVA NPs in Vivo
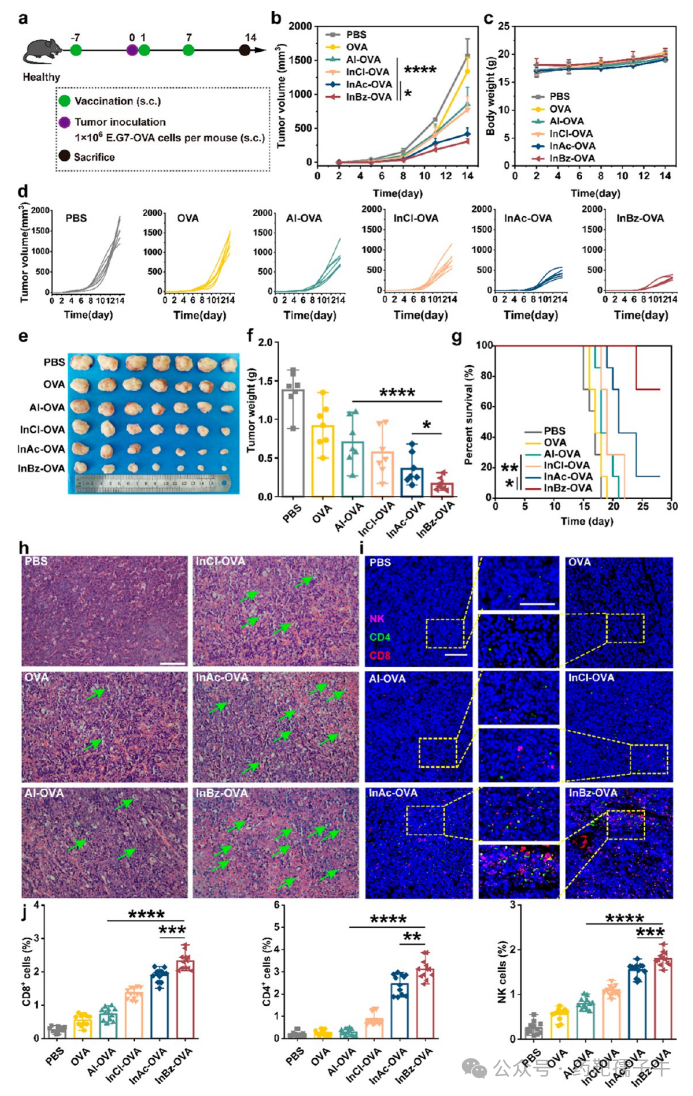
Figure 7 Immune activation effects of PS-OVA NPs in vivo.
8) InBz-OVA NPs’ In Vivo Therapeutic Effects in E.G7-OVA Tumor-Bearing Mice

Figure 8 InBz-OVA NPs’ therapeutic effects in E.G7-OVA tumor-bearing mice.
IV. Conclusion
This study successfully developed a computer-guided chemical modification strategy for the rational design of novel polysaccharide-based vaccine adjuvants. The authors’ innovative approach began with using oligosaccharide fragments that are spatially complementary to the receptor binding site, rather than entire polysaccharide chains, thereby simplifying and accelerating the computational analysis process. Based on this strategy, the authors identified InBz as an efficient polysaccharide-based vaccine adjuvant targeting the TLR4 signaling pathway. Leveraging the intrinsic properties of polysaccharides, the model antigen OVA was efficiently encapsulated in InBz, constructing a vaccine delivery system that provides a structural basis for simultaneously activating APCs. In vitro and in vivo experiments demonstrated that InBz-OVA NPs can efficiently initiate the TLR4 signaling pathway and promote DC maturation, thereby enhancing antigen delivery and presentation efficiency, ultimately eliciting robust antigen-specific humoral and cellular anti-tumor immune responses. Furthermore, the experimental results of InAc and InCl (which have moderate and low affinity for MD2, respectively) as controls were consistent with computational predictions, further validating the reliability of this strategy. The computational analysis and subsequent experimental validation deepened the authors’ understanding of the molecular mechanisms by which polysaccharide derivatives activate TLR4, providing support for the rational design of innovative polysaccharide-based adjuvants. In summary, the authors’ work establishes a computer-guided rational design strategy, laying a solid foundation for the development of polysaccharide-based immunomodulators for tumor vaccines and other immunotherapy applications.
References
Cui Z, Shi C, An R, Tang Y, Li Y, Cao X, Jiang X, Liu CC, Xiao M, Xu L. In Silico-Guided Discovery of Polysaccharide Derivatives as Adjuvants in Nanoparticle Vaccines for Cancer Immunotherapy. ACS Nano. 2025 Jan 21;19(2):2099-2116. doi: 10.1021/acsnano.4c08898. Epub 2025 Jan 9. PMID: 39788571.