Recently, Professor Alexander W. Schuppe’s team from Vanderbilt University reported in J. Am. Chem. Soc. the decarboxylative ligand-to-metal charge transfer (LMCT) reaction of photoinduced Cr (III) carboxylate complexes, applying it to the stereoselective Nozaki-Hiyama-Kishi (NHK) allylation reaction. This method directly utilizes carboxylic acids without the need for external redox reagents, achieving good yields and high enantioselectivity in the synthesis of various high allylic alcohols.Research Background Decarboxylative coupling reactions are an effective strategy for constructing new C−C or C-heteroatom bonds. The radical decarboxylation process can occur at room temperature due to the stability of the generated carbon-centered radical. However, traditional methods require reactive halogen or metalloid reagents. In recent years, photochemical and electrochemical methods have utilized outer-sphere single-electron transfer to oxidize carboxylates, but they face challenges related to redox potential matching and functional group tolerance. In contrast, decarboxylative LMCT, as an inner-sphere process, is less dependent on the redox properties of the metal center. The authors focus on the earth-abundant Cr, whose Cr (II) oxidation state is reactive and can combine with carbon radicals to form Cr (III) reagents without the need for external oxidants. However, the application of Cr’s decarboxylative LMCT in synthetic transformations has not yet been realized, facing challenges such as slow ligand substitution rates and side reactions. Research ContentCondition Screening The research team used (E)-4-cyclohexylbut-3-enoic acid and 3-phenylpropanal as model substrates, screening to find the optimal conditions using the bench-stable Cr pre-catalyst [Cr (dtbbpy)₂Cl₂] Cl (Cr-1), combined with K₂CO₃, Et₃SiCl, and 4Å molecular sieves, under 370nm LED irradiation. At this point, the product yield was 78% with high enantioselectivity. They also found that the type of base, chlorosilane, solvent, and the 2:1 molar ratio of Et₃SiCl to K₂CO₃ were crucial.
Research ContentCondition Screening The research team used (E)-4-cyclohexylbut-3-enoic acid and 3-phenylpropanal as model substrates, screening to find the optimal conditions using the bench-stable Cr pre-catalyst [Cr (dtbbpy)₂Cl₂] Cl (Cr-1), combined with K₂CO₃, Et₃SiCl, and 4Å molecular sieves, under 370nm LED irradiation. At this point, the product yield was 78% with high enantioselectivity. They also found that the type of base, chlorosilane, solvent, and the 2:1 molar ratio of Et₃SiCl to K₂CO₃ were crucial.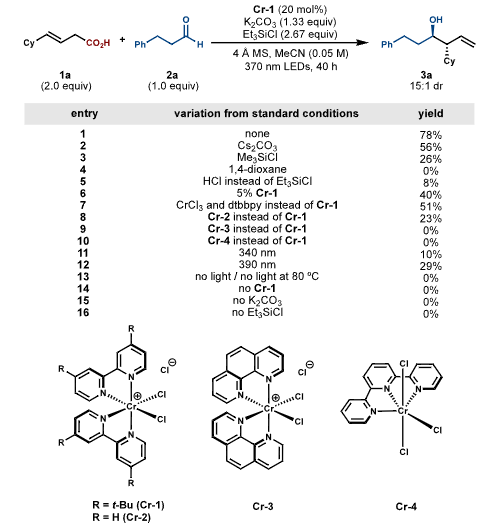 Substrate Expansion The range of aldehyde substrates is broad, including aliphatic, aromatic, and nitrogen-containing heterocycles, as well as alkyl chlorides, esters, furans, amides, and other medicinally relevant substructures. Even aldehydes containing easily deactivated allylic C−H bonds are applicable. For carboxylic acid substrates, those with α or γ substituents that stabilize allylic radicals yield high results. Carboxylic acids with α-heteroatom substituents, ketones, sulfides, and various structural elements, as well as benzo-furans and pyrazoles, can also react. This method can be used to modify drug derivatives and steroids, although certain special cases, such as cyclohexene carboxylic acids due to steric hindrance and aliphatic carboxylic acids due to side reactions, are limited.
Substrate Expansion The range of aldehyde substrates is broad, including aliphatic, aromatic, and nitrogen-containing heterocycles, as well as alkyl chlorides, esters, furans, amides, and other medicinally relevant substructures. Even aldehydes containing easily deactivated allylic C−H bonds are applicable. For carboxylic acid substrates, those with α or γ substituents that stabilize allylic radicals yield high results. Carboxylic acids with α-heteroatom substituents, ketones, sulfides, and various structural elements, as well as benzo-furans and pyrazoles, can also react. This method can be used to modify drug derivatives and steroids, although certain special cases, such as cyclohexene carboxylic acids due to steric hindrance and aliphatic carboxylic acids due to side reactions, are limited.
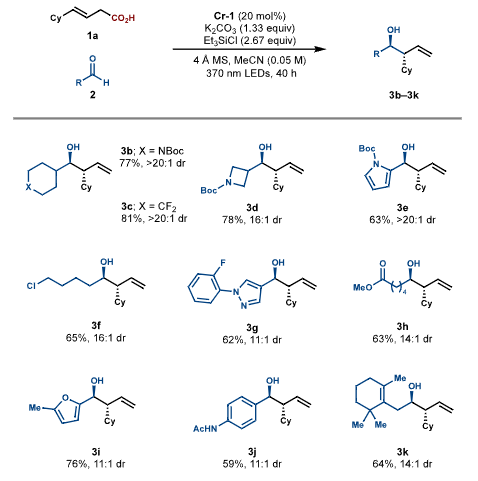 |
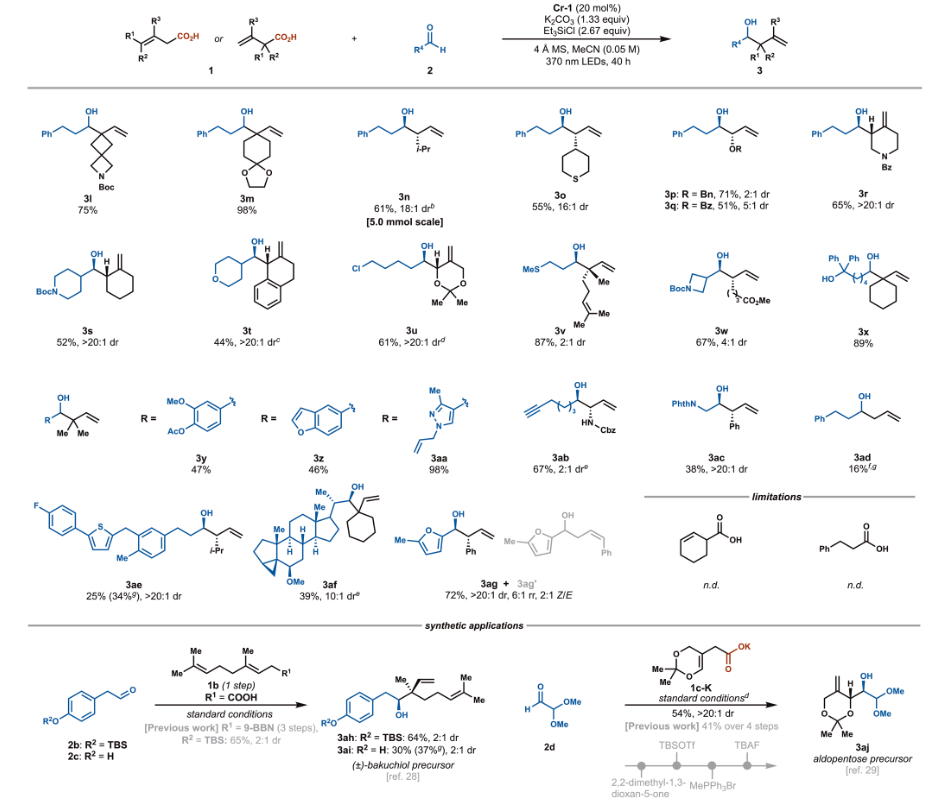 |
Mechanistic Study The research team synthesized different Cr complexes to explore the in situ species, discovering that bidentate pyridine ligands are necessary for the photolysis to generate allylic Cr intermediates. They isolated and characterized the Cr carboxylate complex Cr-7, confirming its catalytic activity. Additionally, through radical trapping and probing experiments, quantum yield measurements, UV−vis spectral monitoring, and 19F NMR experiments, they supported the generation of carboxyl radicals and allylic Cr intermediates during the reaction, ruling out the Cl radical HAT pathway. They proposed that the catalytic cycle involves Cr-1 exchanging with deprotonated carboxylic acids to generate Cr-carboxylate species, with light-induced LMCT producing carboxyl radicals and Cr (II), leading to the formation of allylic radicals that combine with Cr (II), followed by nucleophilic addition of aldehydes and protonation to yield the product.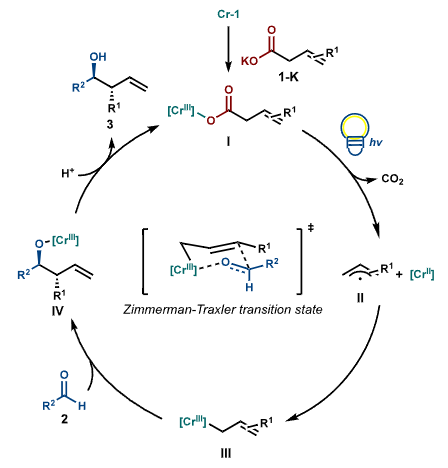 Research Summary This study developed a Cr (III) carboxylate complex LMCT-driven redox-neutral decarboxylative NHK reaction, utilizing carefully designed ligands and additives, allowing a single Cr catalyst to perform both photolysis and C−C bond formation functions, efficiently synthesizing various high allylic alcohols. This provides a new retrosynthetic disconnection approach, and the mechanistic study deepens the understanding of the LMCT process, offering insights for future research.Reference: Unlocking Chromium Decarboxylative Ligand-to-Metal Charge Transfer: Efficient and Redox-Neutral Allylation of Aldehydes Using Carboxylic Acids;J. Am. Chem. Soc. 2025, 147, 26, 22759–22767; https://doi.org/10.1021/jacs.5c04691.
Research Summary This study developed a Cr (III) carboxylate complex LMCT-driven redox-neutral decarboxylative NHK reaction, utilizing carefully designed ligands and additives, allowing a single Cr catalyst to perform both photolysis and C−C bond formation functions, efficiently synthesizing various high allylic alcohols. This provides a new retrosynthetic disconnection approach, and the mechanistic study deepens the understanding of the LMCT process, offering insights for future research.Reference: Unlocking Chromium Decarboxylative Ligand-to-Metal Charge Transfer: Efficient and Redox-Neutral Allylation of Aldehydes Using Carboxylic Acids;J. Am. Chem. Soc. 2025, 147, 26, 22759–22767; https://doi.org/10.1021/jacs.5c04691.