
Adhesive research is an important topic in the field of chemistry, driving advancements across multiple industries from medicine and electronics to construction and automotive. Compared to traditional polymer adhesives, supramolecular adhesives represent an innovative approach that utilizes non-covalent interactions such as van der Waals forces, hydrogen bonding, π-π interactions, and hydrophilic/hydrophobic effects to achieve strong and multifunctional adhesion. The advantage of this supramolecular approach lies in its reversibility and tunability, allowing for precise adjustments of adhesion strength and properties according to different needs. This adaptability makes it particularly suitable for applications requiring stimuli responsiveness, self-healing capabilities, or ease of disassembly and re-adhesion, distinguishing it from traditional irreversible polymer systems.
Despite the broad prospects of supramolecular adhesives, their development still faces significant challenges. The main obstacle is the relatively weak nature of non-covalent interactions, which may lead to insufficient adhesion strength. Furthermore, most supramolecular adhesive systems belong to polymer systems, as achieving high adhesion performance using small molecules as building units is difficult. Nevertheless, researchers are attempting to address this issue through multi-component systems. These systems compensate for their inherent weaknesses by introducing additional non-covalent interactions and optimizing the system by adjusting the ratios of various components. For example, one study optimized the component ratio and chain length by introducing sulfonates into a polyelectrolyte adhesive containing nucleobases and performing counterion exchange, resulting in a 250-fold increase in adhesion strength. Another study developed a supramolecular adhesive with ultra-high adhesion strength through partial coordination of methyl-dopa functionalized castor oil with Fe3+ ions, achieving optimal adhesion under specific component ratios. Despite these advancements, the complexity of multi-component systems often makes understanding the adhesion mechanisms difficult, limiting further improvement opportunities.
Among various supramolecular adhesive systems, single-component small molecule materials are gaining attention due to their simple chemical structure and the ability to establish structure-property relationships more precisely. The clarity of this molecular structure is crucial for understanding adhesion mechanisms and designing materials with specific application properties. However, despite the significant advantages of single-component systems in structural characterization and theoretical modeling, achieving strong adhesion strength remains a major challenge limiting their practical applications.
To address this, the research proposes a synergistic strategy combining supramolecular and covalent polymerization to achieve controllable mechanical performance enhancement of single-component adhesives. This strategy is realized through the design of a custom molecule, BTA-C7, aimed at overcoming the traditional limitations of supramolecular adhesives to achieve performance comparable to conventional polymer systems. The BTA-C7 molecule features a benzene-1,3,5-tricarboxamide (BTA) core, extending with cyano diphenyl vinyl groups, with each arm terminating in a benzo-21-crown-7 ether (B21C7) macrocycle. This design is based on the following considerations: first, the BTA unit facilitates the formation of one-dimensional linear supramolecular polymers through triple hydrogen bonding between amide groups. Second, crown ethers show significant potential in supramolecular adhesives due to their large cavity and electron-donor characteristics. Unlike traditional adhesives, most crown ether-based systems operate at room temperature and exhibit excellent environmental adaptability. Studies have indicated that crown ether adhesives maintain strong performance under extreme conditions (such as low temperatures and underwater), particularly triggering supramolecular polymerization in water, allowing for precise control of adhesion dynamics. Additionally, the cyano diphenyl vinyl group exhibits significant light responsiveness, capable of undergoing self-condensation through [2+2] cycloaddition reactions under UV light, especially in aggregated states or confined environments. Based on these characteristics, BTA-C7 successfully integrates three key features: the BTA group, the B21C7 group, and the cyano diphenyl vinyl unit, expected to enhance performance in self-assembly and covalent crosslinking. Meanwhile, the research also designed two control compounds, BTA-O7 and BTE-C7, which lack specific structural features of BTA-C7 to fully validate the role of each design element in adhesion capability.
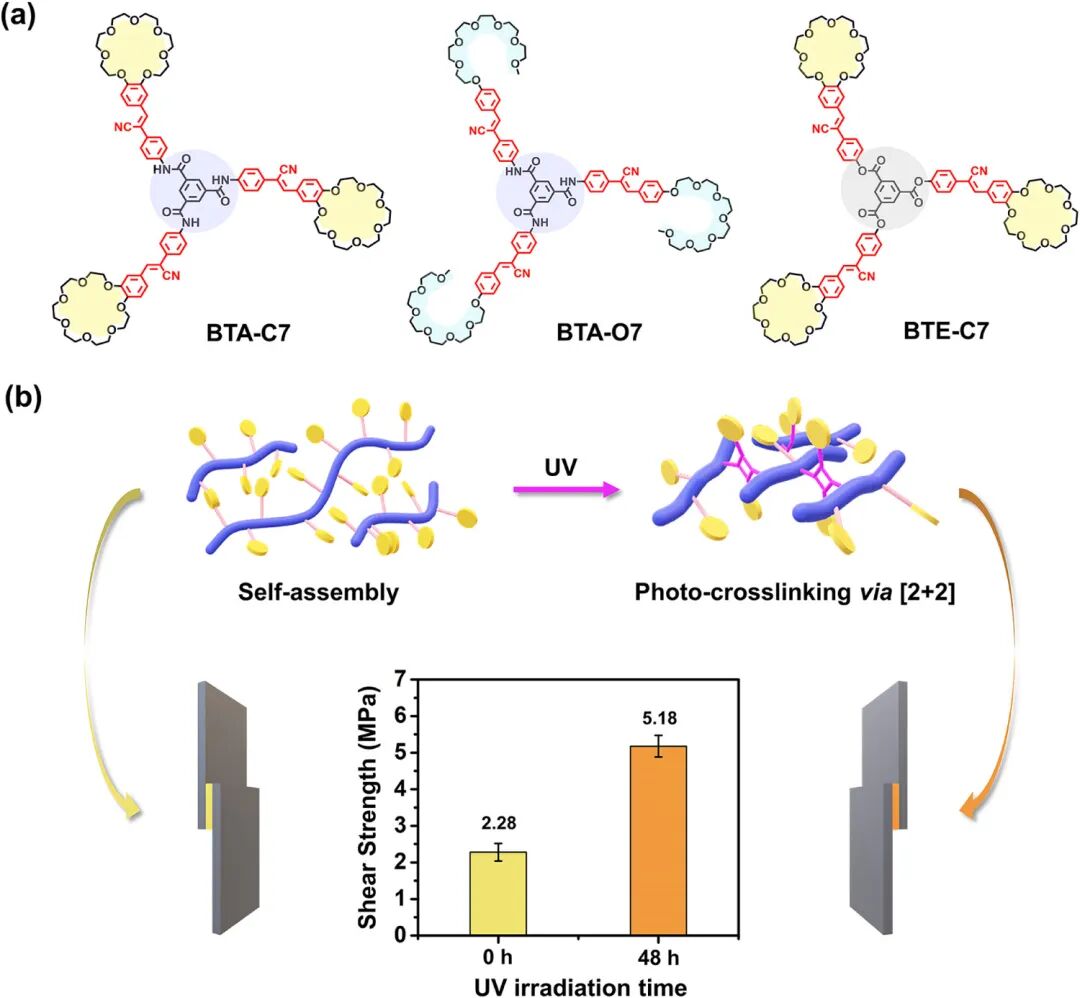
Scheme 1. (a) Molecular structures of the customized compound BTA-C7, model compound BTA-O7, and BTE-C7. (b) Schematic diagram of the synergistic supramolecular and covalent polymerization strategy achieving significant adhesive performance based on BTA-C7.
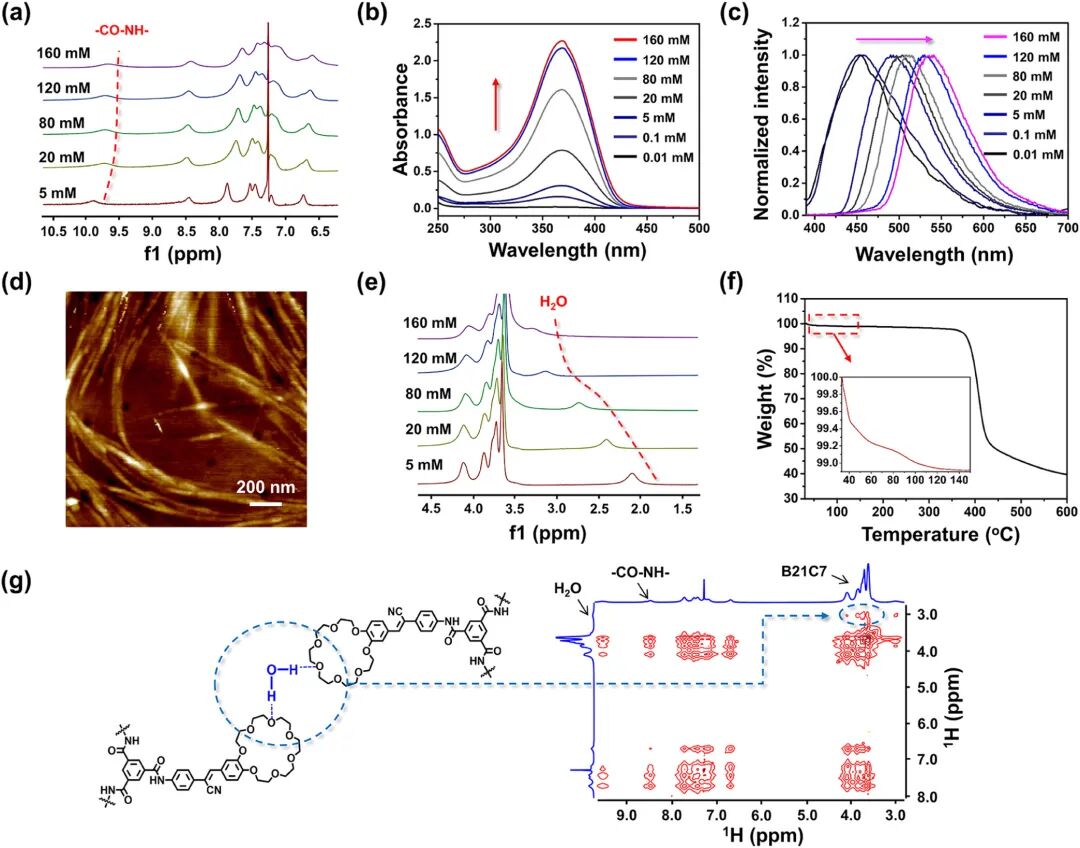
Figure 1. Self-assembly behavior of BTA-C7. (a) Partially concentration-dependent 1H NMR spectra (400 MHz, CDCl3). (b) UV-Vis absorption spectra at different concentrations in CHCl3. (c) Normalized fluorescence spectra at different concentrations (CHCl3, excitation wavelength λex = 365 nm). (d) Atomic force microscopy surface images (160 mM, CHCl3). (e) Partially concentration-dependent 1H NMR spectra (400 MHz, CDCl3). (f) Thermogravimetric analysis (TGA) of BTA-C7 powder. (g) Left: Schematic diagram of intermolecular interactions highlighting hydrogen bonds (blue dashed lines) between terminal B21C7 units and water molecules; right: 1H-1H NOESY spectra (600 MHz, CDCl3).
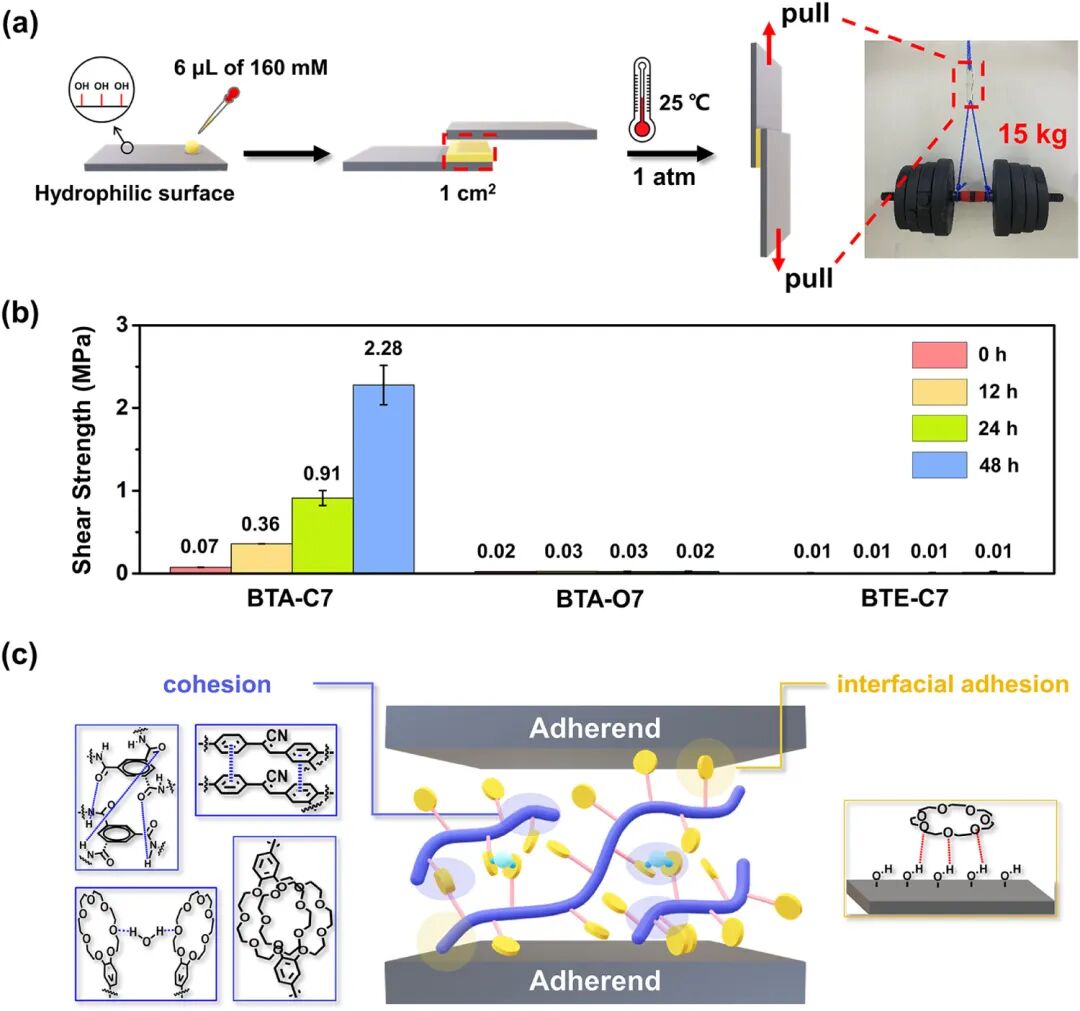
Figure 2. Adhesive performance of BTA-C7 supramolecular polymer. (a) Left: Schematic diagram of the adhesion experiment process. A 6 μL aliquot of 160 mM BTA-C7 solution (dissolved in chloroform) is coated between two glass substrates and cured for 48 hours at 25°C. Right: A photo showing the BTA-C7 adhesive lifting a 15 kg object through a 1 cm² bonding area. (b) Variation of adhesion strength over time: BTA-C7 exhibits a time-dependent mechanical enhancement effect: 2.28 MPa (48 hours curing) compared to nearly no adhesion of model compounds BTA-O7 and BTE-C7. Error bars represent n = 5 repetitions. (c) Schematic diagram of multi-interaction synergistic effects. Cohesive interactions: (i) hydrogen bonds between amide groups, (ii) π-π interactions within the blue-violet phenyl group, (iii) hydrogen bonds between CE rings and water molecules, and (iv) van der Waals forces between CE rings. Interfacial adhesion: hydrogen bonds between CE rings and hydroxyl groups.
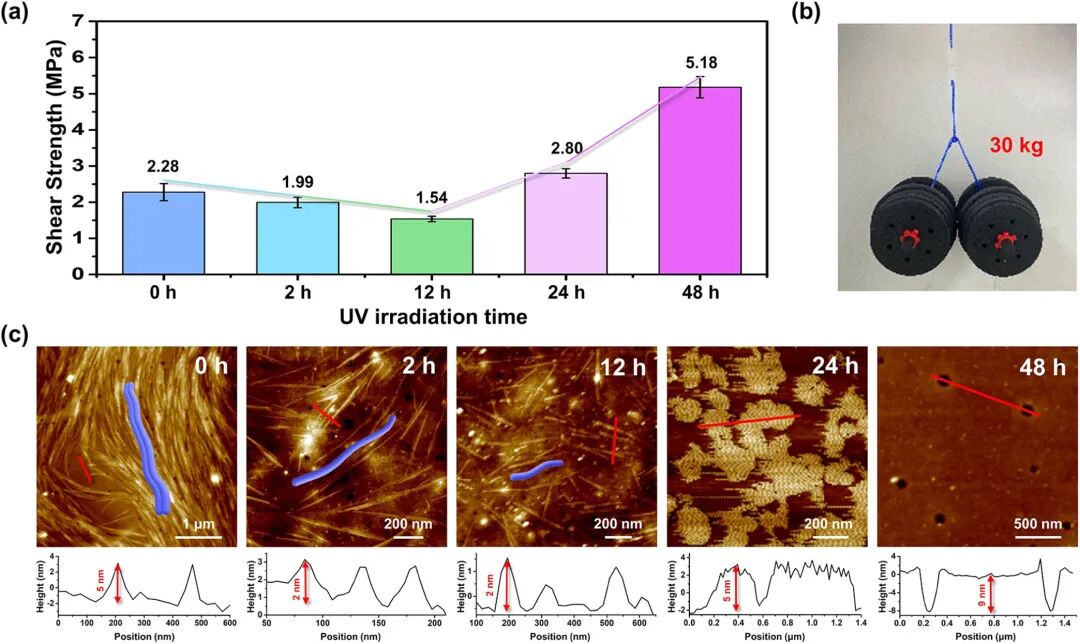
Figure 3. UV-triggered photochemical reaction of BTA-C7 (wavelength 365 nm, power 3.2 mW/cm²). (a) Time-dependent adhesion strength. Error bars represent results from 5 repeated experiments. (b) Macroscopic validation: A photo of the BTA-C7 adhesive (1 cm² bonding area) bearing a 30 kg load after 48 hours of UV exposure. (c) Time-dependent AFM images (160 mM BTA-C7 dissolved in chloroform). Bottom: Cross-sectional images quantifying changes in vertical dimensions.
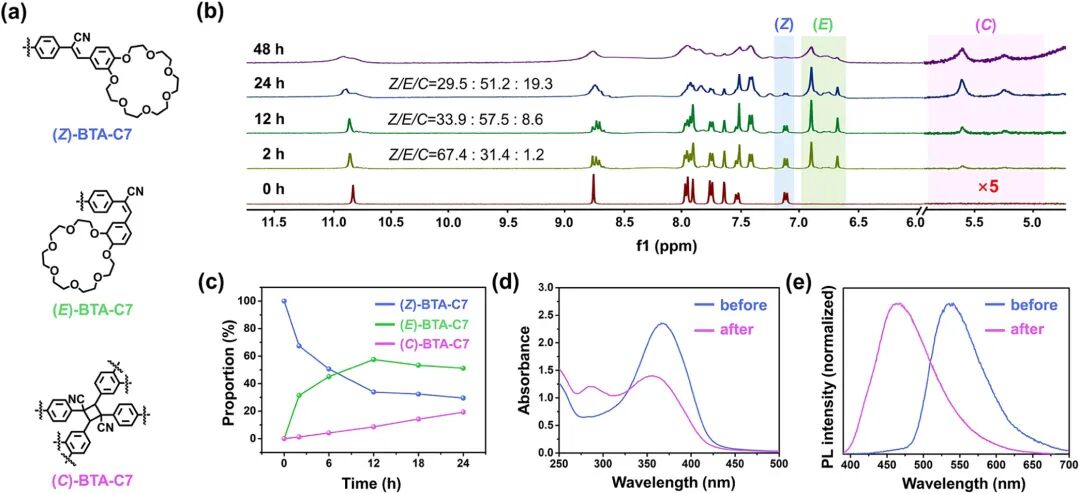
Figure 4. Photochemical reactions of BTA-C7 under UV irradiation. (a) Schematic diagram of molecular structures of (Z)-BTA-C7, (E)-BTA-C7, and (C)-BTA-C7. (b) Time-dependent 1H NMR spectra (400 MHz, DMSO-d6): samples prepared by evaporating CHCl3 from UV-irradiated samples and dissolving in DMSO-d6. (c) Sample ratios at different time points. (d) UV-Vis absorption spectra of BTA-C7 before and after UV irradiation. (e) Fluorescence spectra of BTA-C7 before and after UV irradiation. Experimental conditions: concentration of 160 mM in CHCl3; UV: wavelength 365 nm, power 3.2 mW/cm².
Original link:https://doi.org/10.1021/jacs.5c06302
Please open in the WeChat client