Recently, the team led by Professor Qiu Youai from Nankai University published an article titled “Electrochemical Benzylic C−H Carboxylation” in J. Am. Chem. Soc., reporting a method for achieving benzylic C-H carboxylation reactions through halide-promoted linear paired electrolysis, synthesizing various benzylic carboxylic acids and providing a new green and general method in this field.Research Background Carbon dioxide, as an ideal C1 feedstock, is significant for its conversion into high-value carboxylic acids. Benzylic carboxylic acids are widely present in pharmaceuticals and bioactive molecules. Existing methods for electrochemical synthesis of benzylic carboxylic acids suffer from low atom economy and harsh reaction conditions, creating an urgent need for the development of green and general synthetic methods.Research ContentCondition Screening The research team used butylbenzene as a model substrate to optimize the reaction conditions. It was determined that under a constant current of 20mA at room temperature, using 10mol% NaI as a promoter, nBu4NClO4 as the electrolyte, 1,2-dichloroethane (DCE) as the solvent, with graphite felt as the anode and nickel plate as the cathode, the yield of the target product, benzylic carboxylic acid, could reach 82%.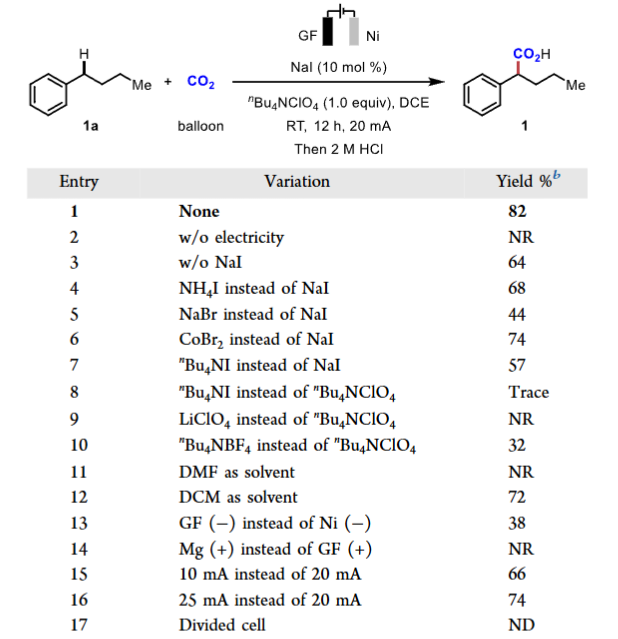 Table 1. Screening of the Reaction Conditions.Substrate Expansion This method shows good compatibility with substrates containing benzylic C-H bonds of varying chain lengths and steric hindrance, selectively cleaving specific C-H bonds. It can effectively construct primary, secondary, and tertiary benzylic carboxylic acids, demonstrating good tolerance for substrates with sensitive functional groups, and is applicable for the synthesis and late-stage modification of bioactive molecules and pharmaceuticals.
Table 1. Screening of the Reaction Conditions.Substrate Expansion This method shows good compatibility with substrates containing benzylic C-H bonds of varying chain lengths and steric hindrance, selectively cleaving specific C-H bonds. It can effectively construct primary, secondary, and tertiary benzylic carboxylic acids, demonstrating good tolerance for substrates with sensitive functional groups, and is applicable for the synthesis and late-stage modification of bioactive molecules and pharmaceuticals.
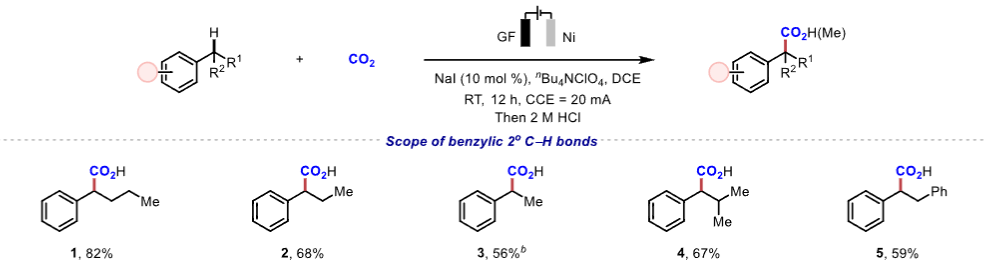 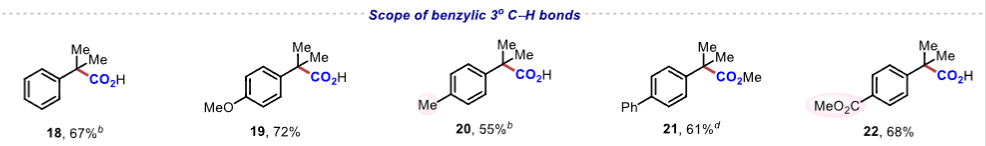 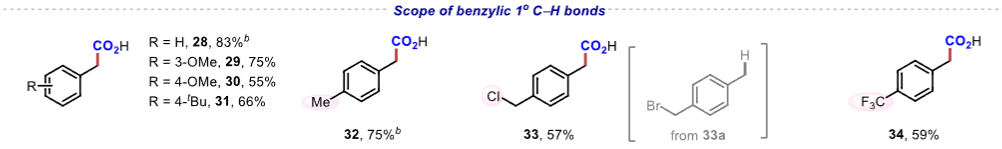 |
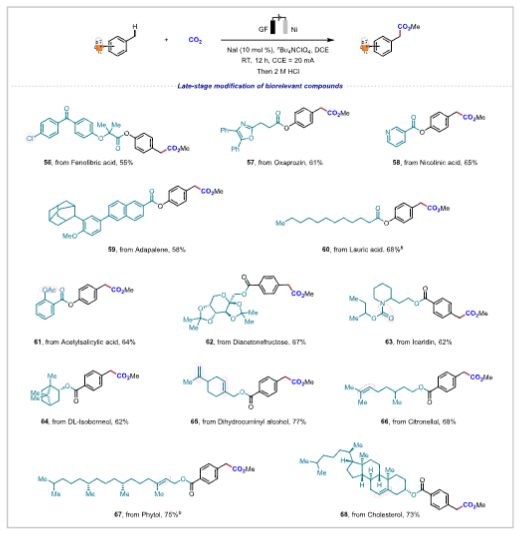
Figure 1. Scope of benzylic C−H bonds.Application Research The reaction conditions can achieve gram-scale reactions and can be used to synthesize drug molecules such as ibuprofen and naproxen, demonstrating high step and atom economy, with significant application value in drug synthesis and late-stage modification of complex compounds.
 |
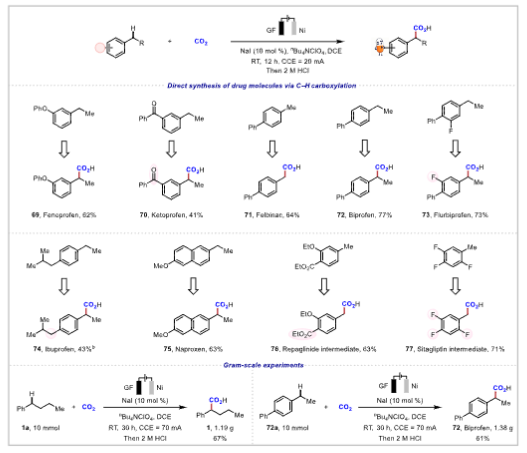 |
Figure 2. Late-stage modification of biorelevant compounds.Mechanistic Study Through radical trapping experiments, deuterated experiments, and cyclic voltammetry studies, a possible reaction mechanism was proposed. DCE gains electrons to form chloride ions, which are oxidized at the anode to generate chlorine radicals that undergo hydrogen atom transfer with the substrate to form benzylic carbon radicals, which subsequently undergo a series of reactions, ultimately resulting in nucleophilic addition with CO2 to yield the product.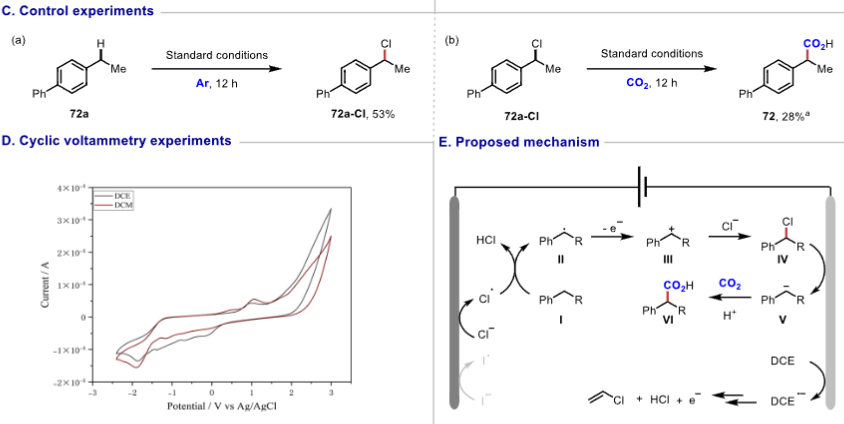 Figure 3. Mechanistic studies and proposed mechanism.Research Summary This study achieved electrochemical benzylic C-H carboxylation reactions, overcoming the inertness of reactants, with high atom utilization, a wide substrate range, and compatibility with various sensitive functional groups. It can be used for drug synthesis and late-stage modification of biologically relevant compounds, providing new pathways for the development of related fields and showing broad application prospects.Reference: Electrochemical Benzylic C–H Carboxylation;J. Am. Chem. Soc. 2025, 147, 16, 13461–13470; doi.org/10.1021/jacs.5c00259.
Figure 3. Mechanistic studies and proposed mechanism.Research Summary This study achieved electrochemical benzylic C-H carboxylation reactions, overcoming the inertness of reactants, with high atom utilization, a wide substrate range, and compatibility with various sensitive functional groups. It can be used for drug synthesis and late-stage modification of biologically relevant compounds, providing new pathways for the development of related fields and showing broad application prospects.Reference: Electrochemical Benzylic C–H Carboxylation;J. Am. Chem. Soc. 2025, 147, 16, 13461–13470; doi.org/10.1021/jacs.5c00259.