Abstract: Compared to other biological agents and small molecule drugs, RNA therapies have significant advantages, including higher safety, lower costs, and broad targeting flexibility, which are rapidly driving the development of the nucleic acid field. This is attributed to breakthroughs in drug delivery, with lipid nanoparticles (LNPs) being one of the most advanced systems clinically. Currently, LNP formulations validated in large populations consist of four types of lipids: ionizable cationic lipids, phospholipids, cholesterol or cholesterol derivatives, and polyethylene glycol (PEG)-lipids. Each lipid is crucial for the normal efficacy of RNA-LNP drugs. Studies have also shown that LNP formulations can induce pro-inflammatory immune responses, which may affect therapeutic outcomes. The mechanisms behind these immune responses and other potential immune reactions to LNP formulations (such as anti-inflammatory or tolerance responses) have yet to be elucidated.
Regarding the mechanisms by which different lipid components in LNP trigger immune responses, we found answers in the following article: The team of Anna Blakney at the University of British Columbia, Canada, published a review in Current Opinion in Biotechnology: “Immune response to the components of lipid nanoparticles for ribonucleic acid therapeutics”, which focuses on how each lipid component in lipid nanoparticles triggers immune responses and impacts the efficacy of RNA vaccines.

Ionizable Cationic Lipids form complexes with negatively charged RNA molecules, and their sensitivity to pH enhances the biocompatibility of LNPs. At normal physiological pH, ionizable cationic lipids are uncharged, reducing their interaction with the anionic membranes of non-target cells. Once LNPs enter endosomes, ionizable cationic lipids become protonated, disrupting the stability of the endosomal membrane and promoting the escape of RNA molecules.Phospholipids play a role in stabilizing the structure of LNPs during particle formation and may also contribute to endosomal escape.Cholesterol or its derivatives stabilize the particles by regulating membrane integrity and rigidity, affecting delivery efficiency and the biodistribution of the particles.PEG-lipids extend the circulation time of LNPs, providing steric hindrance, reducing their size, and preventing aggregation.
Ionizable cationic lipids are key components of LNPs, while phospholipids and cholesterol and derivatives are referred to as auxiliary lipids. Auxiliary lipids can stabilize lipid nanoparticles, enhance transfection efficiency, and prolong the circulation time of the particles. However, despite LNPs being used in large populations, the interaction of auxiliary lipids with the human immune system has largely not been fully studied.
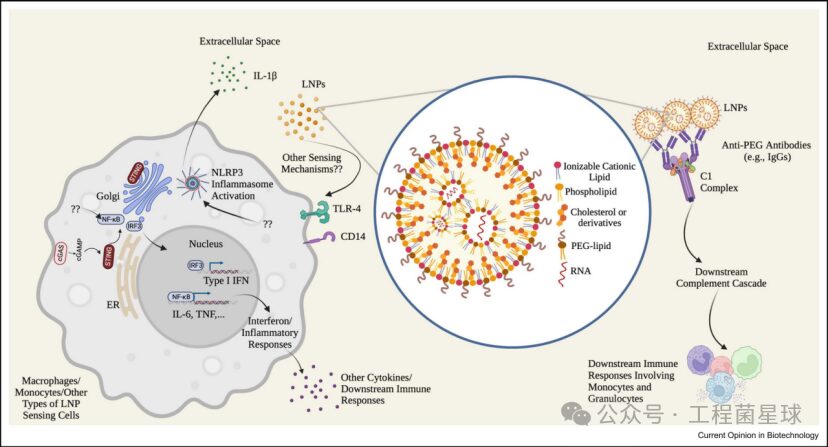
Conventional LNP lipid components and related immune responses
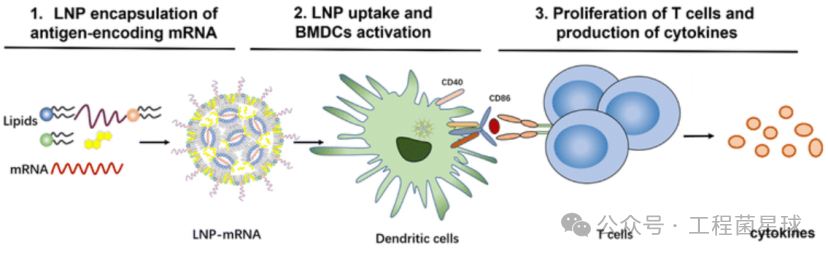
Ionizable cationic lipids are a key component that triggers immune responses in LNPs. Alameh et al. found that LNPs made from specific ionizable cationic lipids as adjuvants for influenza protein subunit vaccines can induce a strong humoral response in immunized mice. However, not all cationic lipids used for nucleic acid delivery can induce a strong humoral response, as they found that LNPs made from non-ionizable cationic lipids had no adjuvant activity.Empty LNPs containing ionizable lipids induce the production of IL-6 in draining lymph nodes of immunized mice, activating follicular helper T cell (Tfh) responses, thereby promoting humoral responses. Moreover, the adjuvant performance of LNPs is superior to widely used MF59-like adjuvant AddaVax.
Similarly, Tahtinen et al. observed that RNA vaccines could stimulate human immune cells to release IL-1 cytokines, primarily IL-1β, depending on the RNA and lipid formulation. IL-1, in turn, triggers a broad spectrum of pro-inflammatory cytokines (including IL-6). Robust IL-1β release was detected when human peripheral blood mononuclear cells were stimulated with modRNA-LNP (SM-102) or empty LNP (SM-102), and co-treatment with R848 (TLR7/8 agonist) and empty LNP (SM-102) further increased IL-1β levels. In contrast, modRNA-LNP (MC3) could not stimulate human peripheral blood mononuclear cells to release IL-1β, even with the addition of R848 in LNP (MC3) being very weak.
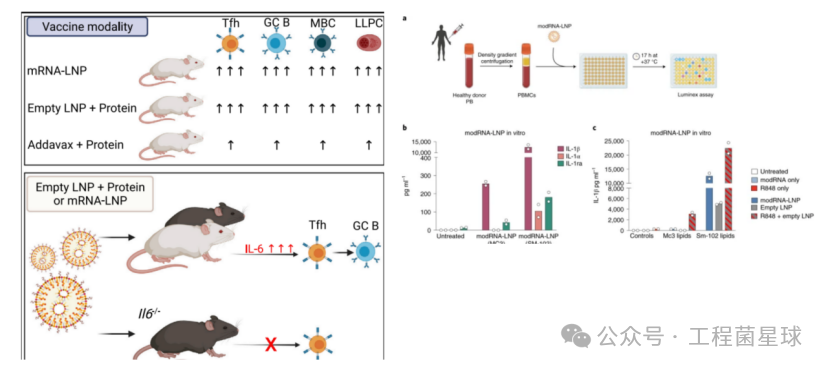
LNPs enhance the efficacy of mRNA and protein subunit vaccines by inducing follicular helper T cells and strong humoral responses (left); modRNA-LNP induces human PBMC to release IL-1β in vitro (right)
The components of ionizable cationic lipids also have a significant impact on local immune responses. Ndeupen et al. found in a mouse model that intradermal injection of empty LNPs lacking ionizable cationic components effectively eliminated skin inflammatory responses (redness and swelling). Flow cytometry showed a large and rapid influx of leukocytes, predominantly neutrophils, in skin samples injected with empty LNPs made from ionizable cationic lipids, which slowly subsided by day 14.
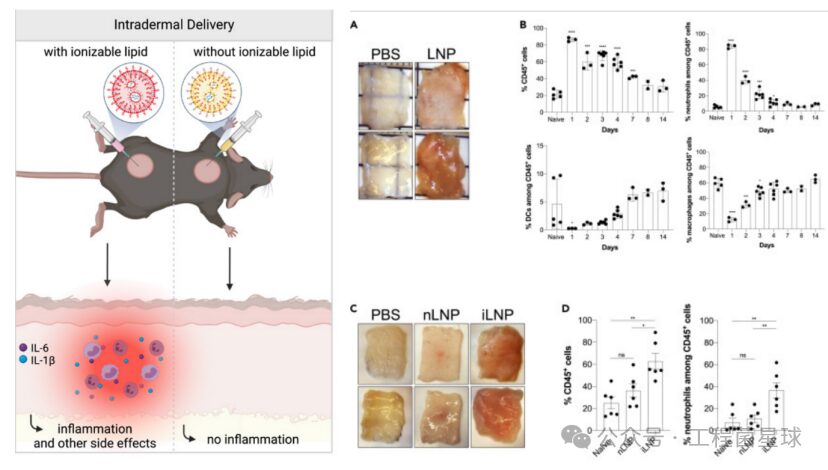 Intradermal injection of LNP triggers a strong inflammatory response
Intradermal injection of LNP triggers a strong inflammatory responseMiao et al. published in Nature Biotechnology: Delivery of mRNA vaccines with heterocyclic lipids increases anti-tumor efficacy by STING-mediated immune cell activation, where they found that compared to empty LNPs made from linear ionizable cationic lipids, empty LNPs made from heterocyclic ionizable cationic lipids upregulated certain activation markers in mouse bone marrow-derived dendritic cells (BMDCs), such as CD40 and MHCII, mediated by the cyclic guanosine monophosphate-adenosine monophosphate synthase (cGAS)-stimulator of interferon genes (STING)-type I interferon (IFN) pathway. This study identified cellular pathways mediating the inflammatory responses triggered by ionizable cationic lipid components and directly linked these responses to the structural characteristics of the lipids. However, not all inflammatory responses induced by ionizable cationic lipids involve the STING pathway. In mouse models, the inflammatory response triggered by LNP configured with C1 ionizable cationic lipids was induced through a TLR-4 pathway independent of STING.
In summary, these studies indicate that the ionizable cationic lipid components in LNP formulations play a critical role in mediating inflammatory responses. These inflammatory responses are associated with the side effects observed in RNA therapies but may also serve as adjuvant effects.
Phospholipids, also known as glycerophospholipids, can be divided into five classes based on their polar head groups: phosphatidylcholine (PC), phosphatidylethanolamine (PE), phosphatidylglycerol (PG), phosphatidylinositol (PI), and phosphatidylserine (PS). Distearoylphosphatidylcholine (DSPC) and dioleoylphosphatidylethanolamine (DOPE) are the most commonly used phospholipids in LNP formulations.
In mammalian cells, PC and PE account for approximately 40-50% and 25% of total phospholipids, respectively. To maintain cellular homeostasis, phospholipids undergo hydrolysis mediated by phospholipases, producing various metabolites, including oxidized phospholipids (both non-enzymatic and enzymatic forms) and lysolipids, which have immunomodulatory effects. For example, oxidized phosphatidylcholine acts as a “damage-associated molecular pattern” (DAMP) and regulates inflammation. Additionally, they modulate signaling through CD14, TLR-4, and the NLRP3 inflammasome, affecting various cellular processes such as phagocytosis, inflammasome activation, and dendritic cell migration. PS also has immunomodulatory effects. PS is present on the surface of apoptotic cells, acting as a “eat me” signal to promote phagocyte uptake. Viruses and other pathogens can present PS on their surfaces to mimic apoptotic cells, facilitating entry into cells and aiding immune evasion.
Researchers have found that LNP formulations containing PS (PS-LNP) can enhance transfection efficiency and increase protein expression of delivered RNA. Researchers added PS to LNPs prepared based on MC3 (PS-LNP) and intravenously injected them into mice. Compared to non-PS-LNPs (where protein expression is mainly distributed in the liver), PS-LNP achieved effective protein expression in both lymph nodes and the spleen. PS-LNP was more distributed in the superficial cervical lymph nodes, and this targeting of secondary lymphoid organs was mediated by monocytes and macrophages.
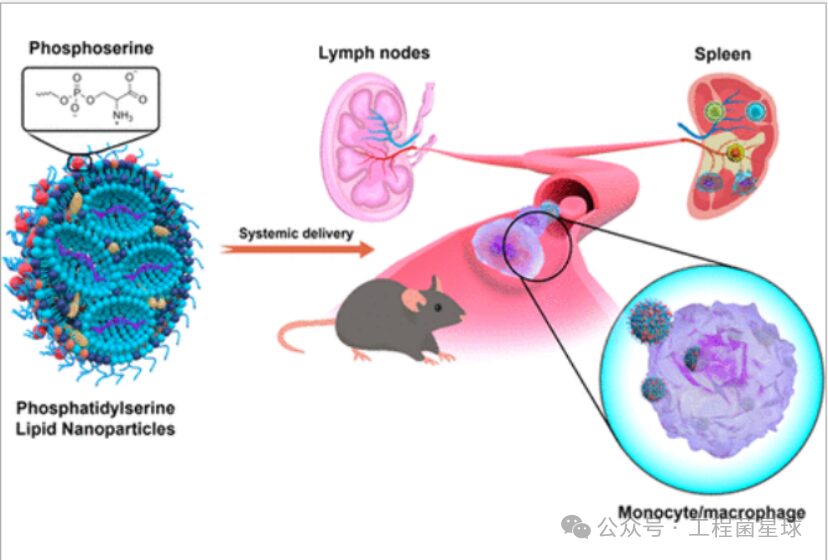
Phosphatidylserine lipid nanoparticles target secondary lymphoid organs
A major clinical complication of treating hemophilia A with recombinant factor VIII (FVIII) is the production of neutralizing antibodies. The complex of FVIII with phosphatidylserine (PS) can reduce the total antibody titer and neutralizing antibody production in hemophilia mice. PS regulates dendritic cell maturation by modulating cytokine secretion (reducing co-stimulatory markers CD86 and CD40 upregulation) and decreases T lymphocyte proliferation, thereby reducing the immunogenicity of FVIII. Nanoparticles containing PS/lysophosphatidylserine can convert immunogens into tolerogenic antigens by inducing tolerogenic dendritic cell production and promoting regulatory T cell (Treg) expansion.
Overall, these studies indicate that phospholipids hold promise as immunomodulatory components in LNP formulations.
Structurally, cholesterol forms and maintains cell membranes. Recent studies have also shown that cholesterol metabolism affects the activity of immune cells, including activation, differentiation, and function. Accumulation of intracellular cholesterol can activate inflammasomes, while oxidized forms of cholesterol play important regulatory roles in immune responses. However, these physiological effects of cholesterol have primarily been studied in the context of atherosclerosis and cardiovascular diseases, and its immune responses as an LNP component in vivo have not been investigated.
Kaur et al. found that compared to liposomes without cholesterol, liposomes containing cholesterol were taken up at a lower rate by macrophages. In terms of immune response, early (12 days post-immunization) IgG responses were reduced with the addition of cholesterol. Cholesterol derivatives can replace cholesterol in LNP-mRNA formulations and may yield higher transfection efficiency and in vivo protein expression in certain experimental contexts. However, it remains unclear whether these observations involve immune pathways.
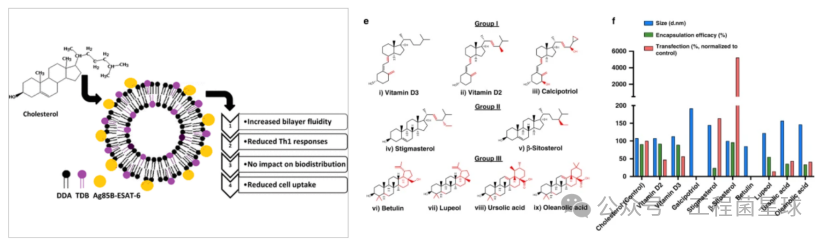
The impact of introducing cholesterol in liposomes on immune responses (left), screening cholesterol derivatives that enhance transfection efficiency of LNP formulations (right)
Some studies maintain a constant amount of ionizable lipids (C12-200 and cKK-E12) and PEGylated lipids, using β-sitosterol to replace cholesterol and varying proportions of the fusogenic lipid DOPE to optimize mRNA delivery efficiency. Different cell lines exhibited varying transfection efficiencies and activation levels for the activation of bone marrow-derived dendritic cells (BMDCs) and T cell proliferation.
Introducing the cholesterol derivative β-sitosterol in LNP stimulates T cell proliferation
Therefore, the physiological roles of cholesterol and its derivatives in the context of LNPs have not been fully defined. Additionally, it has been found that excessive cholesterol in the formulation of LNPs can lead to the formation of insoluble cholesterol crystals in the LNP core. Activation of Toll-like receptors (TLRs) alters cholesterol metabolism in macrophages, leading to increased synthesis and uptake of cholesterol. Direct uptake of cholesterol by LNPs and TLR activation by LNPs both reshape cholesterol metabolism in macrophages and alter their immune functions. Cholesterol’s immune metabolism is involved in both innate and adaptive immune responses, and further studies are needed on how cholesterol components in LNPs promote their immunomodulatory activities.
PEGylation in lipid nanoparticles and other nanocarriers is widely used to enhance their stability and plasma half-life. However, pre-existing or de novo formed PEG antibodies can induce hypersensitivity reactions and accelerate blood clearance by binding to the surface of nanoparticles, leading to activation of the complement system.
Senti et al. found that complement activation triggered by anti-PEG antibodies compromises the integrity of lipid bilayers/surfaces of liposomes, leading to premature drug release or exposure of their mRNA contents to serum proteins. Anti-PEG antibodies can also induce the deposition of complement fragments on the surface of PEGylated lipid nanoparticles and release of liquid-phase complement activation products.
COVID-19 mRNA vaccination increases serum levels of PEG-specific antibodies, and the increase in PEG-specific antibodies correlates with reactivity and the increase of PEG particle-leukocyte complexes in human blood. Moreover, it has been demonstrated that different forms of PEG (e.g., unconjugated PEG, COVID-19 mRNA vaccines, PEG-lipids in other liposomal formulations) activate basophils to varying degrees, and these differences may be related to immune responses associated with allergic reactions.
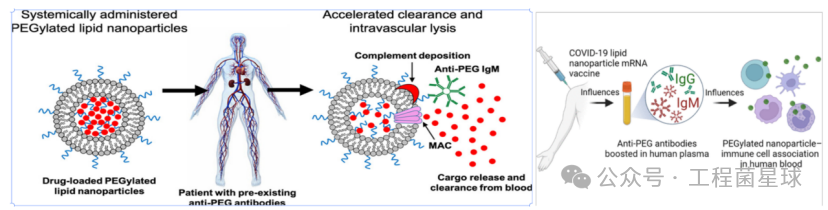
PEG antibodies damage the integrity of PEGylated LNPs via complement activation (left); LNP-mRNA vaccines elevate serum PEG antibody levels (right)
The table below summarizes that each component of LNP formulations approved for clinical use may contribute to overall immunogenicity; however, the detailed mechanisms and interactions behind this remain largely unexplored, especially regarding the overall impact of specific lipid structures (e.g., the number of lipid “tails”, the length of the “tails”, and the linear heads versus cyclic) on the immunogenicity of LNP formulations. Repetitive studies on the mechanisms by which each lipid component triggers immune responses are needed to continuously enhance the safety of RNA-LNP therapies and better optimize the formulation of next-generation delivery systems.
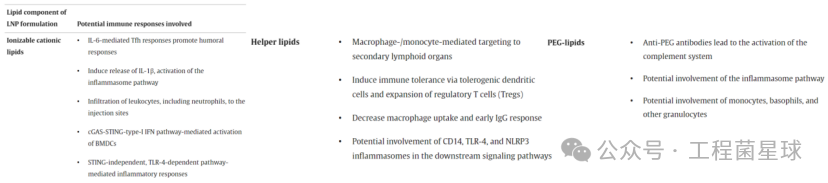
The lipid components of LNP formulations and the potential immune responses involved
Based on pioneering technology and rich experience in the preparation of IVT RNA (mRNA, saRNA, circRNA), Yao Hai Biotechnology can provide global customers with one-stop preparation services for IVT RNA (mRNA, saRNA, circRNA), covering DNA sequence design, IVT RNA preparation, RNA cyclization, RNA purification, RNA lyophilization, LNP encapsulation, RNA quality testing.
Yao Hai Biotechnology also provides various prefabricated plasmid templates (plasmid + bacterial solution), as well as IVT RNA products (liquid/lyophilized powder), LNP finished products, encoding proteins covering reporter genes, target proteins, gene editing proteins, and antigen proteins, including eGFP、mCHERRY、luciferase、IL-2、Cas9、OVA、COVID-19 S protein, etc., to meet different experimental or project needs. For details, please consult Jun Jun: 13380332910 (WeChat same number).
Yao Hai IVT RNA business details:Yao Hai Biotechnology has launched a mRNA research-grade sample custom synthesis one-stop solution


eGFP mRNA and mCherry mRNA achieve high expression in 293T cells
References
[1] Chen SP, Blakney AK. Immune response to the components of lipid nanoparticles for ribonucleic acid therapeutics. Curr Opin Biotechnol. 2023 Dec 19;85:103049. doi: 10.1016/j.copbio.2023.103049.
[2] Alameh MG, Tombácz I, Bettini E, et al. Lipid nanoparticles enhance the efficacy of mRNA and protein subunit vaccines by inducing robust T follicular helper cell and humoral responses. Immunity. 2022 Jun 14;55(6):1136-1138. doi: 10.1016/j.immuni.2022.05.007.
[3] Tahtinen S, Tong AJ, Himmels P, et al. IL-1 and IL-1ra are key regulators of the inflammatory response to RNA vaccines. Nat Immunol. 2022 Apr;23(4):532-542. doi: 10.1038/s41590-022-01160-y.
[4] Ndeupen S, Qin Z, Jacobsen S, Bouteau A, Estanbouli H, Igyártó BZ. The mRNA-LNP platform’s lipid nanoparticle component used in preclinical vaccine studies is highly inflammatory. iScience. 2021 Dec 17;24(12):103479. doi: 10.1016/j.isci.2021.103479.
[5] Miao L, Li L, Huang Y, et al. Delivery of mRNA vaccines with heterocyclic lipids increases anti-tumor efficacy by STING-mediated immune cell activation. Nat Biotechnol. 2019 Oct;37(10):1174-1185. doi: 10.1038/s41587-019-0247-3.
[6] Luozhong S, Yuan Z, Sarmiento T, et al. Phosphatidylserine Lipid Nanoparticles Promote Systemic RNA Delivery to Secondary Lymphoid Organs. Nano Lett. 2022 Oct 26;22(20):8304-8311. doi: 10.1021/acs.nanolett.2c03234.
[7] Kaur R, Henriksen-Lacey M, Wilkhu J, et al. Effect of incorporating cholesterol into DDA:TDB liposomal adjuvants on bilayer properties, biodistribution, and immune responses. Mol Pharm. 2014 Jan 6;11(1):197-207. doi: 10.1021/mp400372j.
[8] Patel S, Ashwanikumar N, Robinson E, et al. Naturally-occurring cholesterol analogues in lipid nanoparticles induce polymorphic shape and enhance intracellular delivery of mRNA. Nat Commun. 2020 Feb 20;11(1):983. doi: 10.1038/s41467-020-14527-2.
[9] Zeng Y, Escalona-Rayo O, Knol R, et al. Lipid nanoparticle-based mRNA candidates elicit potent T cell responses. Biomater Sci. 2023 Jan 31;11(3):964-974. doi: 10.1039/d2bm01581a.
[10] Estapé Senti M, de Jongh CA, Dijkxhoorn K, et al. Anti-PEG antibodies compromise the integrity of PEGylated lipid-based nanoparticles via complement. J Control Release. 2022 Jan;341:475-486. doi: 10.1016/j.jconrel.2021.11.042.
Recommended Reading
[Yao Wen Interpretation] Understanding the Development Status of Veterinary mRNA Vaccines at Home and Abroad
[Yao Wen Interpretation] New Technologies for the Design, In Vitro Preparation, and Purification of Circular RNA (circRNA) Sequences
[Yao Wen Interpretation] Sanofi’s Latest Publication: Analysis of mRNA-LNP Finished Products: How to Extract mRNA from LNP
[Yao Wen Interpretation] BioNTech’s Publication: The Design of mRNA Influenza Vaccines: The Impact of Cap Structures and Modified Nucleotides (m1Ψ)
Yao Hai Biotechnology’s services cover the preparation and commercialization of samples from pre-IND to clinical phase III in areas such as “recombinant proteins/vaccines, plasmids/mRNA, nanobodies”, dedicated to creating a comprehensive, customized one-stop service platform for CRO/CDMO/MAH. It has a 20,000 square meter workshop that meets GMP requirements and a 3,000 square meter R&D center, providing customized services ranging from 50L to 2000L according to customer needs. For related information, please contact official customer service Yao Junjun at: 13380332910 (same WeChat number).



