
iNature
Sentinel lymph node biopsy is of great significance in cancer treatment. Due to the lack of specific and sensitive optical probes, early detection and precise excision of metastatic lymph nodes (LNs) still face challenges.
On December 16, 2024, a research paper titled “Spatiotemporal Mapping of Lymphatic Metastases in Gastric Cancer Using Tumor-Trackable and Enzyme-Activatable Near-Infrared Fluorescent Nanoprobes” was published online in ACS Nano by Han Fanghai from Jinan University/Zhongshan University along with Huang Jiaguo and Xu Weiping from Zhongshan University.
This study reports a metastatic LN reporter gene (MLR) with activatable optical output for precise spatiotemporal mapping of gastric cancer lymphatic metastasis.
MLRs are self-assembled entities that contain a mixed amphiphile with a lipophilic tail and tumor-targeting ligands or fluorescent moieties, which are surrounded by a switch cleaved by tumor-specific β-galactosidase (β-gal). After draining to the LN, in the presence of disseminated tumor cells, MLRs selectively activate their near-infrared fluorescence. In an in situ gastric cancer mouse model, the representative reporter gene MLR1 distinguished macro/micro metastatic LNs from benign LNs and was able to spatially detect early skipping LN metastasis. This active sensing mechanism is comparable in sensitivity and specificity to flow cytometry analysis. In surgical specimens from patients, MLR1 distinguished cancerous tissues and metastatic LNs from normal tissues and benign LNs within one hour.Therefore, this study developed NIRF nanoprobes that are easily detectable in lymph node metastases in gastric cancer patients, highlighting a probe for understanding metastatic progression.

Gastric cancer (GC) is the second most common malignant gastrointestinal tumor and the fourth leading cause of cancer death, with approximately 1.1 million new cases and 760,000 deaths globally in 2020. Due to a lack of specific symptoms in the early stages, about 70% of GC patients are diagnosed at an advanced stage, often accompanied by lymph node (LN) metastasis. Although LN metastasis is an important adverse prognostic factor closely related to survival rates, its dissemination pathways in GC remain poorly understood. Non-invasive imaging techniques, such as positron emission tomography (PET), computed tomography (CT), and magnetic resonance imaging (MRI), have been used for preoperative staging but lack sensitivity in detecting early lymphatic metastasis. The lymphoscintigraphy with 99m-technetium (99 mTc) is a gold-standard clinical method for detecting and excising sentinel LNs, but it poses radioactive risks. Despite the effectiveness of sentinel LN biopsy (SLNB), identifying metastatic LNs within fibrofatty tissue remains challenging, with a relatively high false-negative rate of 13-52%. Therefore, there is an urgent need for alternative methods for non-invasive early detection of metastatic LNs and guiding surgeons for precise excision.
Fluorescence imaging enables real-time, non-invasive visualization of dynamic biological pathological processes in live subjects with high sensitivity.Clinically, indocyanine green (ICG) is used as a signal-to-background ratio (SBR) tracer for intraoperative real-time visualization of lymphatic vessels and sentinel LNs. However, due to non-specific interactions in the human body, its “always-on” fluorescence may lead to false-positive results, hindering accurate tracking of cancer metastasis. Although other probes with passive (such as polymer-coated nanoprobes, gold nanoclusters, or quantum dots) or active (such as IRDye800CW-conjugated bevacizumab, cetuximab, or panitumumab) tumor-targeting capabilities have been explored, they exhibit non-specific biodistribution, resulting in low signal-to-noise ratios and insufficient differentiation between macro/micro metastatic LNs and benign LNs. In contrast, activatable probes that generate specific responses to tumor biomarkers (such as overexpressed enzymes or abnormal acidity) provide higher sensitivity for the precise excision of metastatic foci. However, few studies have reported activatable probes for early detection of disseminated cancer cells within metastatic LNs. Furthermore, the patterns of lymphatic metastasis in GC are highly complex, with abnormal lymphatic drainage patterns frequently leading to skipping LN metastasis. Previous studies primarily focused on investigating the frequency of sentinel LN metastasis, and the temporal dynamics (time-dependent manner) of tumor cell dissemination within the lymphatic network and the anatomical locations (spatial-dependent manner) of metastatic LNs remain unclear.
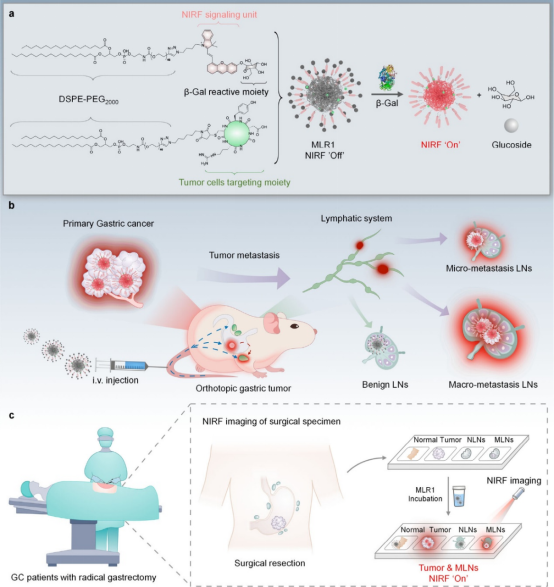
Figure 1 Schematic diagram of tumor-trackable and enzyme-activatable NIRF nanoprobes for spatiotemporal localization of metastatic LNs (adapted from ACS Nano)
This study reports a tumor-trackable and enzyme-activatable NIRF nanoprobes (MLR) for spatiotemporal localization of metastatic LNs.MLRs are self-assembled nanocomplexes that consist of two chemically modified 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-poly(ethylene glycol) (DSPE-PEG) derivatives. Each derivative has a lipophilic DSPE tail for efficient LN delivery and is functionalized at the other end with a cyclic arginine-glycine-aspartic acid peptide (cRGDyC) targeting integrins, which is overexpressed as a tumor recognition motif in cancer, or d-galactose residues that lock the half-cyanine NIRF fluorophore, which can be cleaved by the tumor biomarker β-galactosidase (β-Gal) as a sensing motif. Because the cage-like phenolic groups inhibit the electron donor capability, MLRs are essentially non-fluorescent. They emit fluorescence only in the presence of increased β-Gal in disseminated tumor cells, thus able to specifically identify lymph node metastasis. Meanwhile, the authors described the construction and screening of MLR1-3 in a subcutaneous gastric tumor model. MLR1 exhibited the best performance in tumor targeting and sensing, further optimized in an in situ gastric tumor model for spatiotemporal localization of metastatic LNs, distinguishing macro and micro metastatic LNs from benign LNs, and able to early detect skipping LN metastasis patterns in a spatially dependent manner.Finally, the authors demonstrated the application of this probe in identifying cancerous tissues and metastatic LNs in collected specimens during radical gastrectomy, showcasing the potential of this probe in assisting early diagnosis of metastatic LNs clinically.
Reference:
https://pubs.acs.org/doi/10.1021/acsnano.4c12915

—END—
The content is original from 【iNature】 public account,
Please indicate the source as 【iNature】
Add WeChat Group
iNature gathers 40,000 researchers and doctors in life sciences. We have formed 80 comprehensive groups (16 PI groups and 64 PhD groups), and also established specialized groups in relevant fields (plants, immunology, cells, microbiology, gene editing, neurology, chemistry, physics, cardiovascular, oncology, etc.).Warm reminder: Please indicate when joining the group (format such as school + major + name, if you are a PI/professor, pleaseindicate as PI/professor, otherwise it will be directlyassumed as a doctoral student, thank you).You can first add the editor’s WeChat ID (love_iNature), or long press the QR code to add the editor, and then join the relevant group. Serious inquiries only.

Submission, cooperation, and reprint authorization matters
Please contact WeChat ID:13701829856 or email:[email protected]
If you find this article interesting, please click here!