Due to their adaptability to the surrounding environment, soft microrobots have developed various therapeutic applications in the medical field, including vascular clearance, cell transport, and drug delivery. However, most soft microrobots cannot quickly enter, retrieve, and maintain their original position once the external driving field is removed. To address this, Guannan He from China Medical University and Associate Researcher Jiao Niandong from the Shenyang Institute of Automation, Chinese Academy of Sciences have introduced a soft magnetic microrobot for targeted drug delivery that can be transported into the body through a catheter and anchored to tissues. This microrobot features a dual-layer adhesive body, consisting of a mussel-inspired hydrogel layer and an octopus-inspired magnetic structure layer. Based on the differences in adhesion of the hydrogel layer in air and water, entry and retrieval are completed with the help of medical catheters. The microrobot can achieve multiple motion modes under an applied magnetic field and while adhering to underwater tissues. The adaptability and recoverability of the microrobot were demonstrated using a gastric model. Coupled with ultrasound (US) imaging, the feasibility of operating the microrobot in the isolated small intestine was shown. Furthermore, a highly efficient targeted drug delivery method was identified using a fluorescence imaging system. Thus, the soft magnetic microrobot proposed in this study has great potential for medical applications.
Related research was published on October 25, 2023, in 《Small》 under the title “Catheter-Assisted Bioinspired Adhesive Magnetic Soft Millirobot for Drug Delivery”.
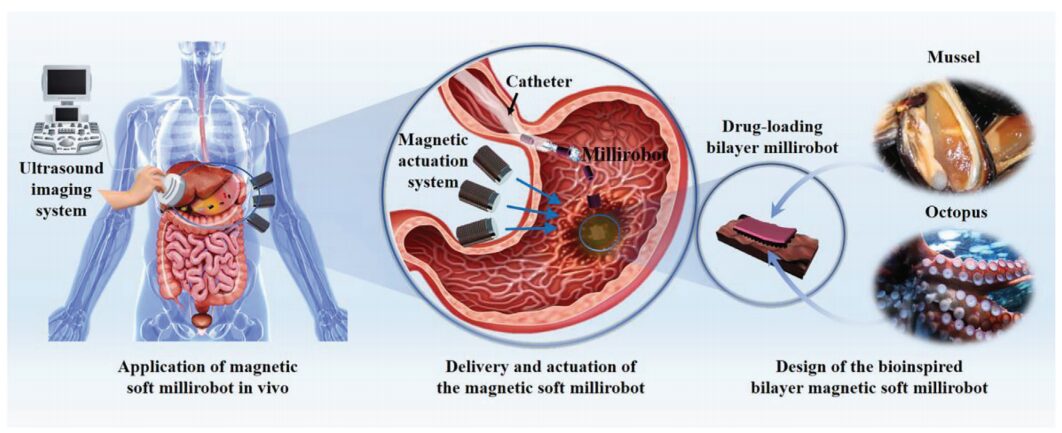
Schematic diagram of the bio-inspired magnetic microrobot principle and its biomedical applications
This article develops a soft adhesive magnetic microrobot using PDA hydrogel and magnetic particle-doped polydimethylsiloxane (PDMS). The microrobot adapts to its environment by stretching itself in a reconfigurable manner. As shown in the schematic, the microrobot effectively enters deep and narrow spaces in the human body through an adhesive “fast track” catheter, detaching from the catheter without causing damage. This prevents direct contact with the complex fluid environment of the human body and allows the microrobot to traverse rugged and complex organs.
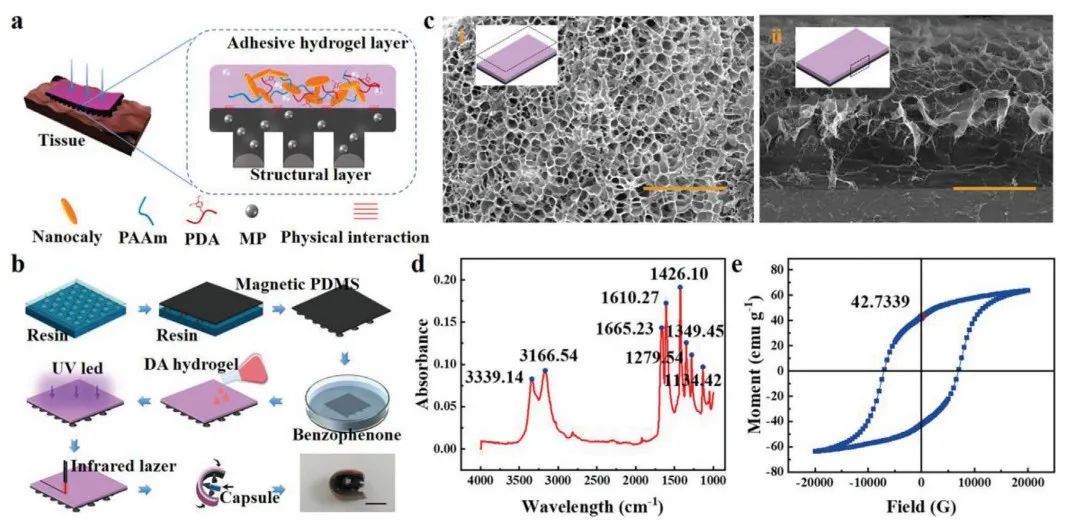
Figure 1 Manufacturing and characterization of multilayer microrobots
Figure 1a shows the detailed structure of a soft magnetic microrobot. The multilayer microrobot consists of a magnetic PDMS adhesive layer and a bio-adhesive hydrogel layer. The magnetic suction cup array structure layer was made by curing the structure magnetic PDMS using a 3D printed resin mold (Figure 1b). Scanning electron microscope (SEM) images (Figure 1ci) show that the hydrophilic hydrogel of the microrobot has a compact, uniform porous structure, providing sufficient space for drug loading, allowing the hydrogel to have good drug loading capacity (Figure 1ci). The side profile shows that the hydrophilic hydrogel is tightly bonded to the magnetic PDMS interface (Figure 1cii). At 1665.23 cm−1, a significant peak associated with the amide C═O indicates the presence of acrylamide (Figure 1d). The magnetic intensity of the magnetic PDMS layer is then described (Figure 1e). The hysteresis loop shows significant remanence and coercivity, allowing the magnetic microrobot to be effectively driven under an applied magnetic field.
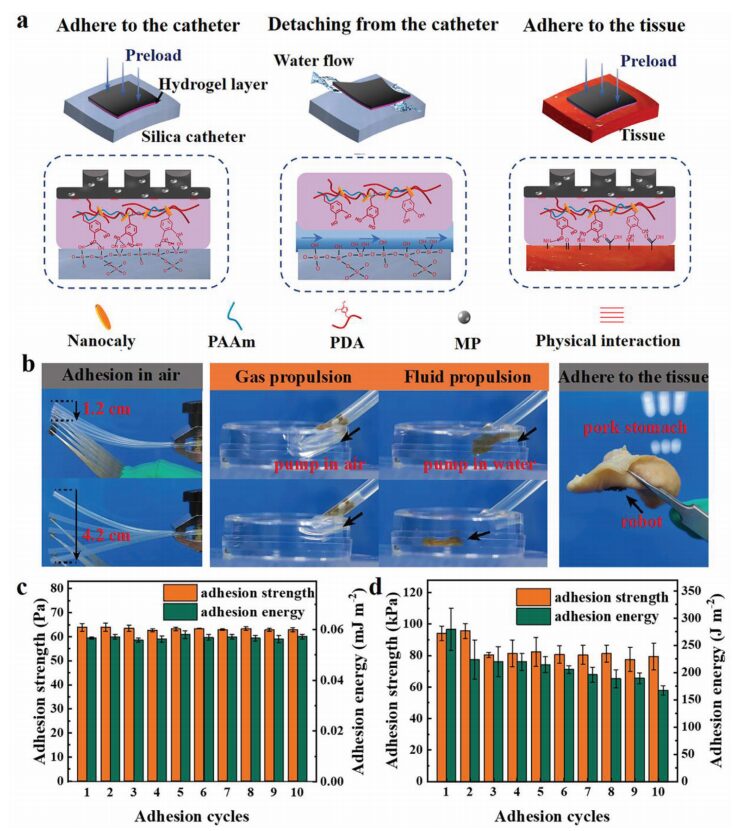
Figure 2 Adhesive mechanism and performance of the multilayer microrobot adhesive layer
The microrobot can release by injecting physiological saline into the catheter. Figure 2a shows the bio-adhesive layer of the double-molecular-layer microrobot adhering to the catheter, separation induced by current flushing, and adhesion to biological tissue. Figure 2b displays the adhesion of different layers of the microrobot to air and the adhesion to the surface of the pig’s stomach. The hydrogel exhibits high adhesion strength and energy in air, maintaining strong adhesive performance during the initial repeated adhesion process (Figure 2c). However, the adhesion strength and energy significantly decrease in water (Figure 2d). This study’s method utilizes the excellent adhesion of the hydrogel in air and the low adhesion in water to achieve loading and release of the microrobot.
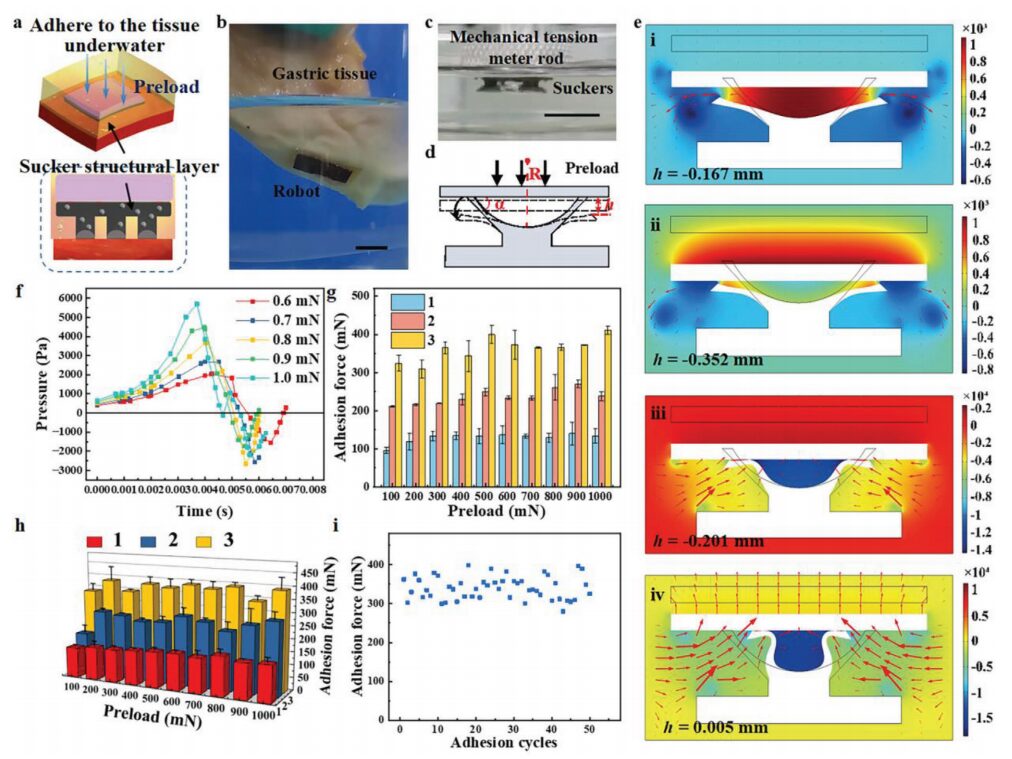
Figure 3 Adhesive mechanism and performance of the multilayer microrobot magnetic suction cup structure layer
This study utilizes a 3D printed template to design and fabricate a magnetic suction cup structure layer, which adheres to biological surfaces under the influence of magnetic force (Figure 3a). The microrobot can adhere well to underwater tissue surfaces and maintain adhesion under vibrational disturbance, mimicking the daily activities of the patient (Figure 3b). By controlling the tension meter, the adhesive force of the microrobot’s suction cup structure layer is measured under preloaded conditions; the deformation of the suction cup during the downward pressure of the rod is shown in Figure 3c. The deformation of the suction cup during the downward pressure rod process is shown in Figure 3d. Figure 3e presents the simulation results of the suction cup and surrounding fluid state during the downward pressure of a glass slide under a 600 mN preload. Figure 3f shows the variation of internal pressure in the suction cup under different preload conditions, with the preload reversing at 0.005 s. The adhesion force formed by the glass substrate under a preload greater than 100 mN is similar, with an average adhesion force of 120 mN for a single suction cup (Figure 3g). The same conclusion is drawn for adhesion to soft biological tissue underwater (Figure 3h). Additionally, it has been verified that the suction cup structure layer exhibits good repeatability (Figure 3i). After 50 repeated underwater adhesions, the adhesion force of the microrobot showed no downward trend.
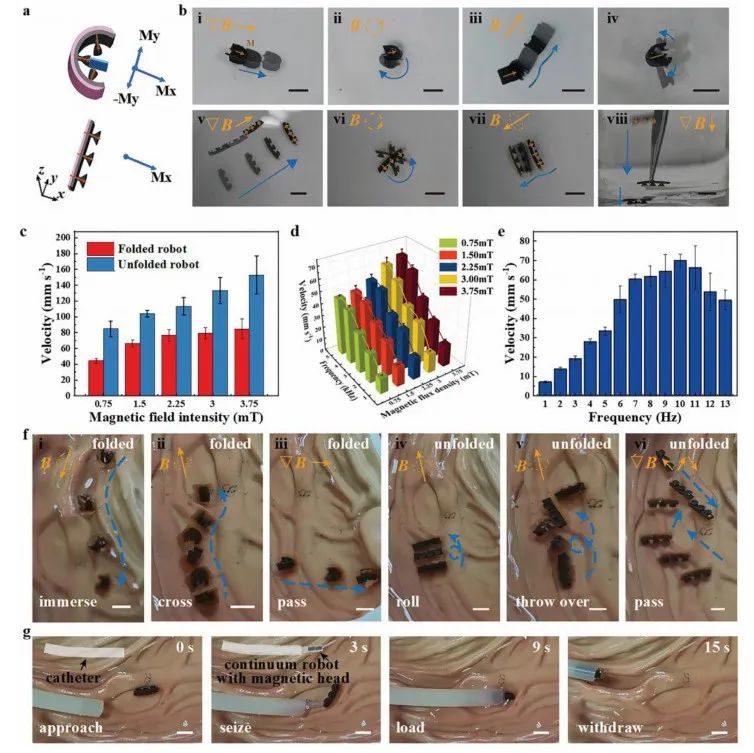
Figure 4 Various magnetic motion modes and in vitro experiments
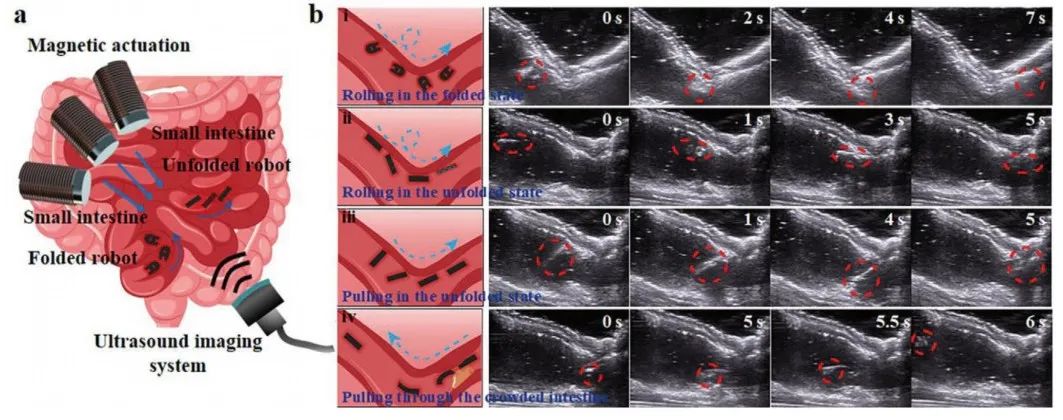
Figure 5 Various magnetic motion modes and in vitro experiments of the microrobot
In addition to being delivered through catheters, the microrobot can also be magnetically guided to the target area. Due to the magnetization from the strong external magnetic field applied during the curing of PDMS, the microrobot exhibits ferromagnetic properties (Figure 4a). In Figure 4b, the blue arrows indicate the trajectory of motion during magnetic controlled movement, and the yellow symbols represent the external driving magnetic field (gradient, rotating, and rolling magnetic fields). The speed of the microrobot in folded and unfolded states increases with the strength of the external magnetic field (Figure 4c). During rolling motion, the speed of the microrobot in folded state increases with the increase of external magnetic field strength and shows an increasing trend with frequency in the range of 1-5 Hz (Figure 4d). In the unfolded state, its rolling speed also shows an upward trend with frequency in the range of 1-10 Hz, but uncontrolled stalling occurs when the frequency exceeds 10 Hz, leading to a decrease in speed (Figure 4e). The microrobot in the folded state can roll into water in a humid environment (Figure 4f i). The microrobot can roll through the stomach folds and roll flat (Figure 4f ii, iv, v). Figures 4f iii, vi show that the microrobot can quickly translate underwater relying on magnetic force. The process of extracting the microrobot using a magnetic continuum robot through a catheter is shown in Figure 4g.
The magnetic microrobot confirmed its biocompatibility in vitro within the pig intestine (Figure 5a). The microrobot was initially placed at the left end of the pig small intestine; Figure 5b i shows a folded microrobot rolling along the intestinal wall under a rolling magnetic field, passing through the turning point of the small intestine. As the capsule expands, the microrobot folds itself, but it can still freely roll forward along the intestinal wall (Figure 5b ii). The microrobot translates under the influence of the magnetic field, changing its translation direction after rotating at a certain angle through a turn (Figure 5b iii). Although the intestine is congested with food debris and other objects, the microrobot can still pass through the intestine under the influence of a large magnetic field due to its flexibility and thinness (Figure 5b iv). By combining real-time clinical imaging equipment, the motion of the microrobot in vivo can be observed, which is a fundamental prerequisite for the microrobot’s medical application in vivo.
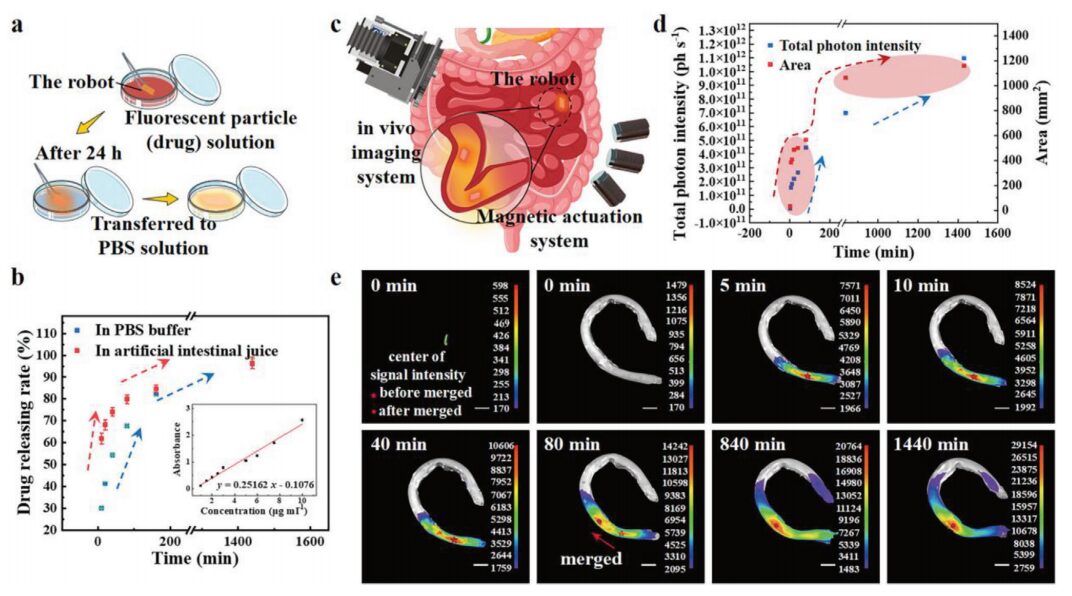
Figure 6 In vitro experiments of this microrobot and efficient targeted drug release application
Using rhodamine fluorescent nanoparticles as markers to visualize the state of drug diffusion (Figure 6a). A UV-visible near-infrared spectrophotometer was used to calibrate the relationship between drug concentration and absorbance, and to measure the concentration of released drugs (Figure 6b). Fluorescence images and bright field photos were obtained using an in vivo imaging system (Figure 6c). The total photon intensity and detectable diffusion area of the fluorescent particles are shown in Figure 6d. Figure 6e indicates that the fluorescent signal of the drug loaded in the microrobot can be detected. Therefore, the microrobot, combined with clinical imaging equipment, has considerable potential for in vivo medical applications.
In summary, this study proposes a new microrobot design for targeted, long-term drug release in vivo. The advantages of adhesive materials and structures are applied to the field of robotics, addressing key issues that cannot be ignored in the clinical application of medical microrobots. A soft magnetic robot was designed in this study, which can be delivered through a catheter to the human body and manipulated to anchor to the target area of tissues for medical applications. The robot consists of a mussel-inspired hydrogel layer and a magnetic suction cup structure layer, thus it has a dual-layer adhesive surface with different adhesion mechanisms. This study utilizes the variable adhesion strength of the adhesive hydrogel in air and underwater, allowing the microrobot to achieve fast, non-damaging delivery through catheters. Strong adhesion in air prevents separation during delivery, while weaker adhesion in water ensures controlled, non-damaging release. The magnetic suction cup structure layer enables the robot to navigate internally in various motion modes under an external magnetic field, and the robot can also adhere to tissue surfaces after negative pressure expansion. This study simulated the biological environment within the pig intestine and demonstrated the feasibility of internal operation of the robot using US imaging. Additionally, the targeted drug release capability of the microrobot in vivo was verified in conjunction with the in vivo imaging system. Biodegradable materials are undoubtedly the preferred choice for designing robots for more therapeutic scenarios. Future improvements in robot structure and materials can achieve more effective motion modes and enhanced degradability.
Source:
https://doi.org/10.1002/smll.202306510
Join Group for Discussion/Submission/Recommendation
Focusing on the research direction of regenerative medicine, the EFL public account has established an “Academic Exchange Group“. Scan the QR code below to add the editor’s WeChat for group discussions. Submissions/Recommendations can be sent to the submission email~
If you found this article helpful, please click on the “Follow,” “Share,” “View,” “Like” icons to support EFL’s content creation and let us share cutting-edge information with more researchers~