In the 1966 American science fiction film “Fantastic Voyage,” several American doctors shrink a submarine to a size smaller than a red blood cell and send it through blood vessels into the brain to clear a clot blocking the brain, ultimately saving a scientist’s life.
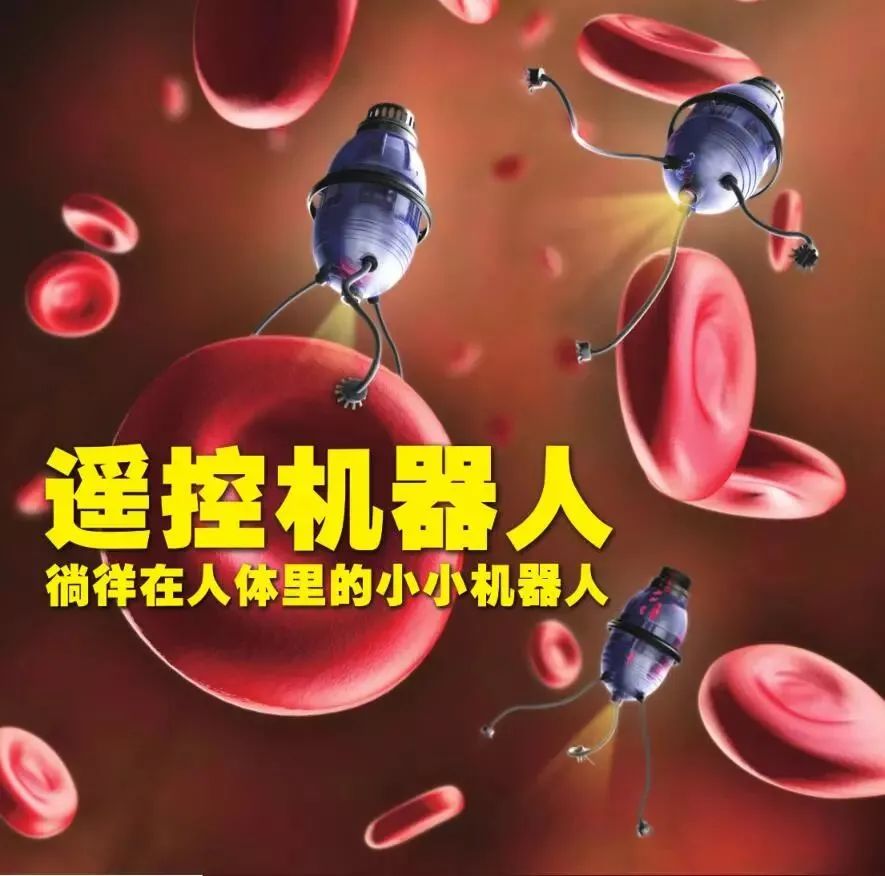
From this film, we can see that the concept of using micro-robots for medical treatment has long been established. In the past, this idea could only be found in science fiction works; however, with the advancement of technology, the dream of implanting micro-robots into the human body may soon become a reality.
In the near future, when we visit the hospital, doctors may not need to prescribe medication or perform surgery but instead inject micro-robots into our bodies. These robots can freely navigate within the human body, clearing arteries blocked by plaque, conducting live tissue examinations, or treating cancer and tumors from the inside.

The Challenge of “Miniaturization”
The ideal size for medical micro-robots is comparable to human cells, and unlike traditional medical methods such as surgery and catheter insertion, micro-robots cause almost no damage to human tissue. By targeting specific sites within the body and delivering drugs directionally, micro-robots can significantly reduce side effects.
Despite the numerous benefits of medical micro-robots, achieving this is not easy. The biggest difference between micro-robots and traditional robots is their extremely small size. The “miniature” size is a double-edged sword, bringing advantages while also imposing serious limitations. Micro-robots that can be injected into the human body are too small to easily integrate any power sources, sensors, or computer circuits. Features that larger robots possess, such as mobility and artificial intelligence, are difficult to achieve in micro-robots. For example, consider the miniature submarine at the beginning; a submarine the size of a cell cannot have motors installed, rendering its propellers useless. Moreover, traditional mechanical drives (like underwater propellers) cannot be applied to internal robots due to the need to protect human tissue. How the submarine moves through thick blood becomes the biggest issue.
Additionally, at the micro scale, surface area-related effects become more pronounced, further limiting the movement of micro-robots. Every object has volume and surface area, and the ratio of surface area to volume is crucial. Generally, the smaller the object, the larger this ratio becomes. Thus, at the microscopic scale, effects proportional to surface area (like air resistance) have a greater impact, while effects related to volume (such as gravity and inertia) play a smaller role. For instance, some insects are tiny and have a large surface area-to-volume ratio, allowing them to fall from heights unharmed because they are significantly affected by air resistance during their descent, greatly reducing their terminal velocity.
Besides the challenges posed by small size, the biodegradability and biocompatibility of micro-robots are also key factors. Small foreign objects should not remain permanently in the human body and should not provoke severe immune responses, so any materials entering the body need to be strictly selected.
Activating Micro-Robots
Faced with these challenges, scientists have devised various methods to activate micro-robots. The first is acoustic driving, where oscillating sound waves act on the liquid surrounding the micro-robots, creating pressure differences on either side of the liquid, causing the micro-robots to move. Another method is chemical driving, which utilizes microbubbles generated from chemical reactions to provide propulsion.
Live cells that have energy and mobility can also power micro-robots, such as certain bacteria and muscle cells. Engineers can combine living cells with artificially manufactured micro-devices and control them remotely by altering environmental conditions like temperature, acidity, and light. However, the problem with this method is that it can only be used in controlled environments, and changes in the human body’s environment cannot be too drastic.
The most popular form of robot propulsion is magnetism. Engineers embed magnetic materials within the robots and manipulate the micro-robots using an external magnetic field. To produce a strong magnetic field, engineers place movable magnets or electromagnetic coils outside the human body, as the magnetic field can harmlessly affect the body. Changing the direction and gradient of the magnetic field applies force and torque (the force that causes an object to rotate) to the micro-robots, guiding them along the desired trajectory; the changes in the direction and gradient of the magnetic field determine how the micro-robots move.
Rotation, Sliding, and Rolling
In addition to the activation methods for micro-robots, the ways they move during experiments vary. Since most of the human body’s environment is liquid, micro-robots need to have the ability to move in fluid environments. Some researchers look to nature for solutions and notice that many microorganisms propel themselves using flagella (protein appendages with movement functions found on certain bacteria). Spiral micro-robots designed based on flagella can rotate and advance like a screw drill under the drive of a rotating magnetic field. However, this rotational movement is suitable for liquid environments but not for the rough, viscous surfaces inside the human body (like the stomach lining).
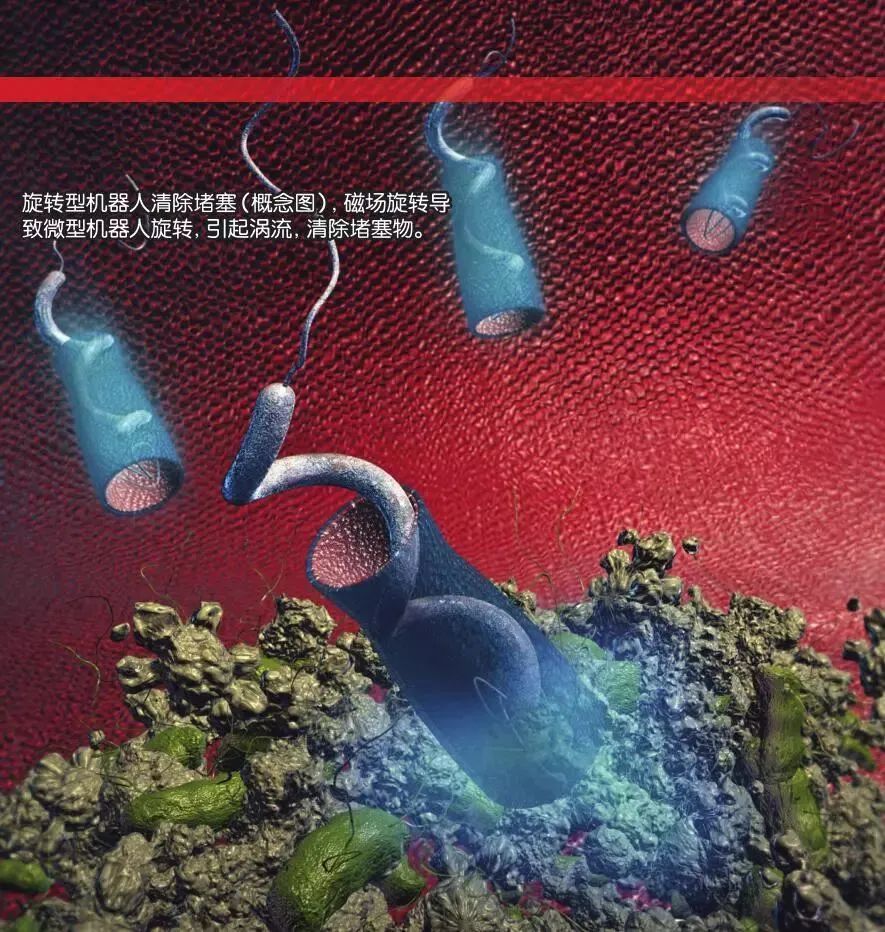
The most common movement method is using a magnetic gradient to make micro-robots slide; the magnetic gradient pulls magnetic objects toward areas of stronger magnetic fields. However, on rough surfaces, sliding under the influence of a magnetic gradient encounters significant resistance, such as friction. Therefore, although sliding performs well in laboratory settings, it is not suitable for practical applications.
There are also other movement methods, such as rolling (flipping). Since rotating magnetic fields can be used to rotate artificial flagella, they can also be used to rotate thin, flat objects. When a long, flat micro-robot is subjected to magnetic torque, it flips over, with one side of the micro-robot firmly “gripping” the surface due to friction, while the other side flips forward, allowing the micro-robot to begin moving. Through this movement method, micro-robots can freely traverse the stomach’s rugged surface.

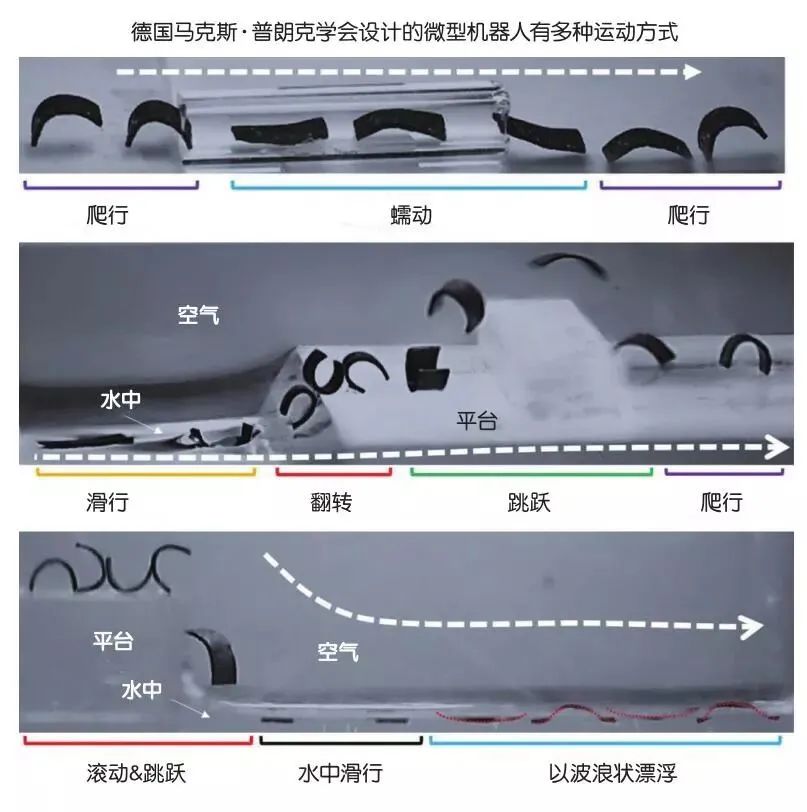
Micro-robots can possess one movement method or multiple methods. For example, in 2016, researchers at ETH Zurich in Switzerland created spiral micro-robots made from biocompatible hydrogels and magnetic nanoparticles, which moved in a rotating manner in liquid environments under the influence of a magnetic field. In 2018, micro-robots designed by the Max Planck Society in Germany could roll, jump, or crawl on uneven surfaces, as well as swim in liquid environments. Similar to the spiral micro-robots, these micro-robots embedded magnetic particles within elastic silicone sheets measuring only 4 millimeters in length (less than half the width of a fingernail), and researchers controlled the robots’ movement through an external magnetic field.
Cooperative Teams and Task Execution
To achieve better application effects for micro-robots, scientists hope to enable a group of robots to work together, much like an ant colony. For example, multiple micro-robots can collaborate to carry more drugs into the body. However, achieving cooperation among micro-robots still faces challenges, as current magnetic field control technology cannot individually direct robots to move separately; all robots are controlled by the same large magnetic field. Currently, scientists are researching micro-electromagnetic field technology for small-scale driving, hoping to coordinate the control of multiple micro-robots in the future.

Once we can perfectly control and move micro-robots, the next challenge will be to enable the robots to perform various complex tasks. Currently, micro-robots can only perform simple actions, such as pushing and grabbing objects. To complete the anticipated medical tasks, the operational capabilities of micro-robots must be enhanced.
With the advancement of miniaturization technology, in the not-too-distant future, teams of micro-robots will swim through blood vessels, exploring every corner of the body and transporting therapeutic drugs and repairing cells along the way.