
From J. Am. Chem. Soc.
Hi, everyone! I wonder if you still remember that I shared an article titled 【J. Am. Chem. Soc.】Me₃N•BH₃—— Cu Catalyzed Halogenated Alkane + Primary Amide N-Alkylation, which achieved amide N-alkylation by generating alkyl radicals through XAT halogen transfer. However, in an earlier discussion, we learned that the 【J. Am. Chem. Soc.】Photocatalytic Quasi-Suzuki Coupling can also produce alkyl radicals by combining morpholine radicals with alkyl boronic esters.
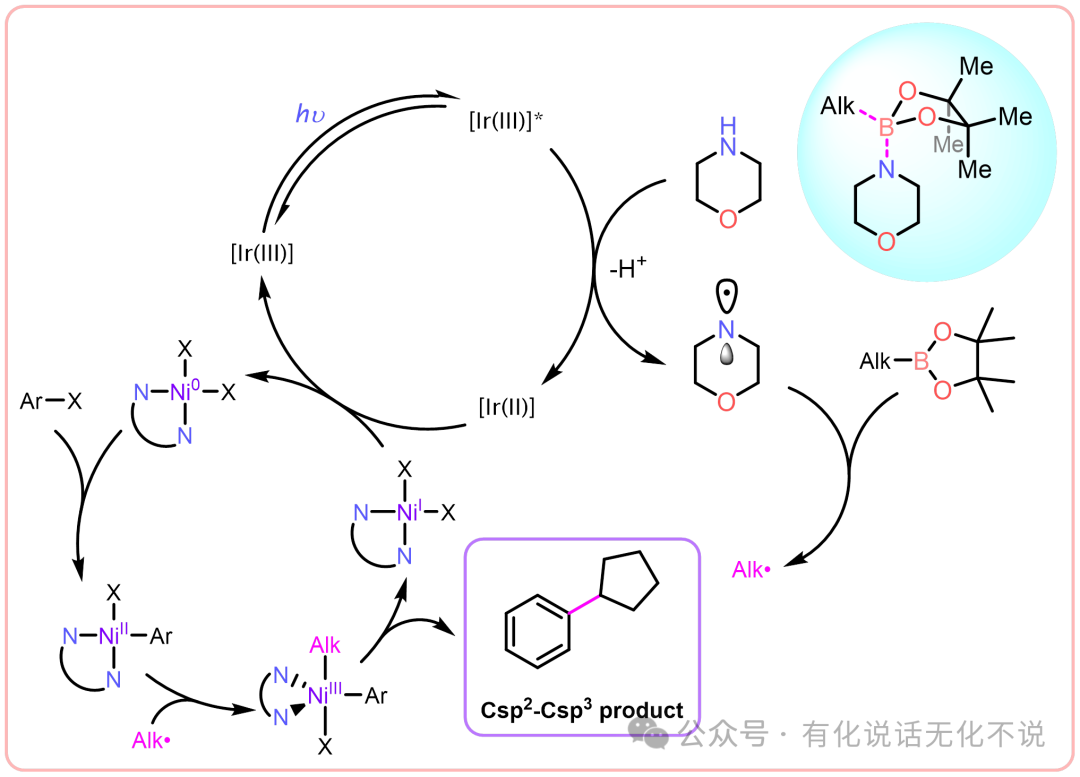
Can this strategy be applied to N-alkylation? Of course, this issue I would like to recommend:
Goodbye Morpholine Radical
——Cu Catalyzed N-Alkylation
From J. Am. Chem. Soc.
⭐Highlights⭐
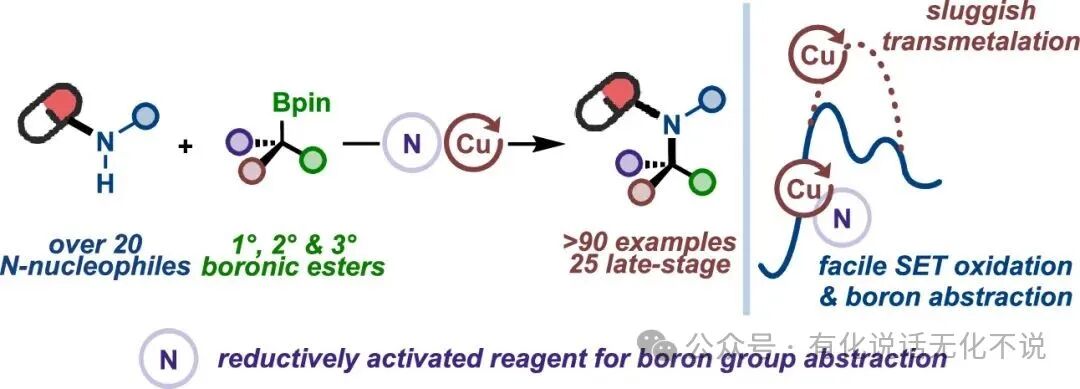
【1】This method utilizes morpholine-based benzoic acid esters as activating agents for alkyl boronic esters, achieving Cu-catalyzed N-alkylation reactions;
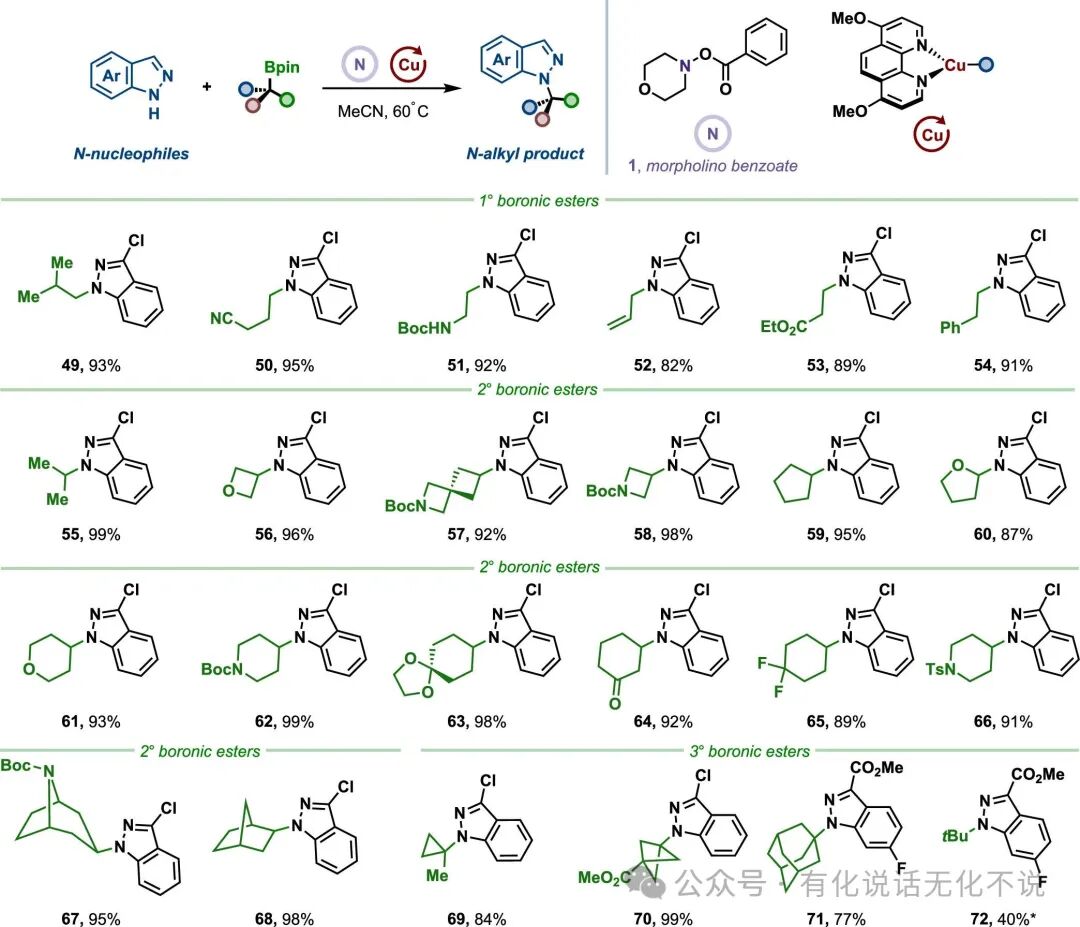
【2】The method has a wide substrate scope for alkyl boronic esters, allowing various primary, secondary, and tertiary boronic esters to participate in the reaction. Notably, MeO₂C-BCP-BPin achieved a yield of up to 99% under this method, realizing 3-chloroindole-N₁-alkylation;
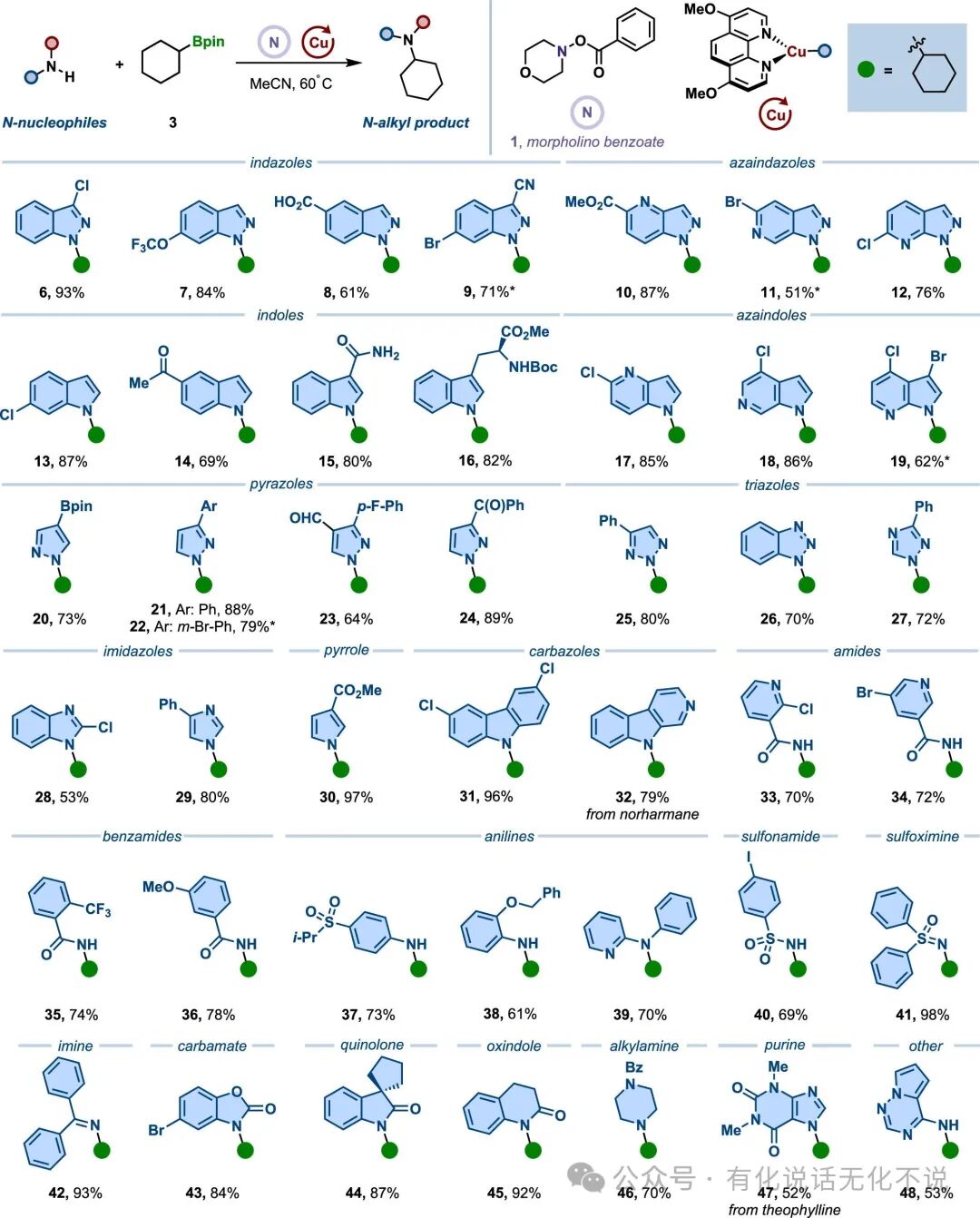
【3】The method exhibits good functional group tolerance, accommodating aromatic carboxylic acids, aromatic aldehydes, aryl boronic esters, aryl bromides, aryl chlorides, trifluoromethoxy, trifluoromethyl, cyano, halogens, benzyl, acetal, ester, amide, sulfonamide, ether, and other functional groups;
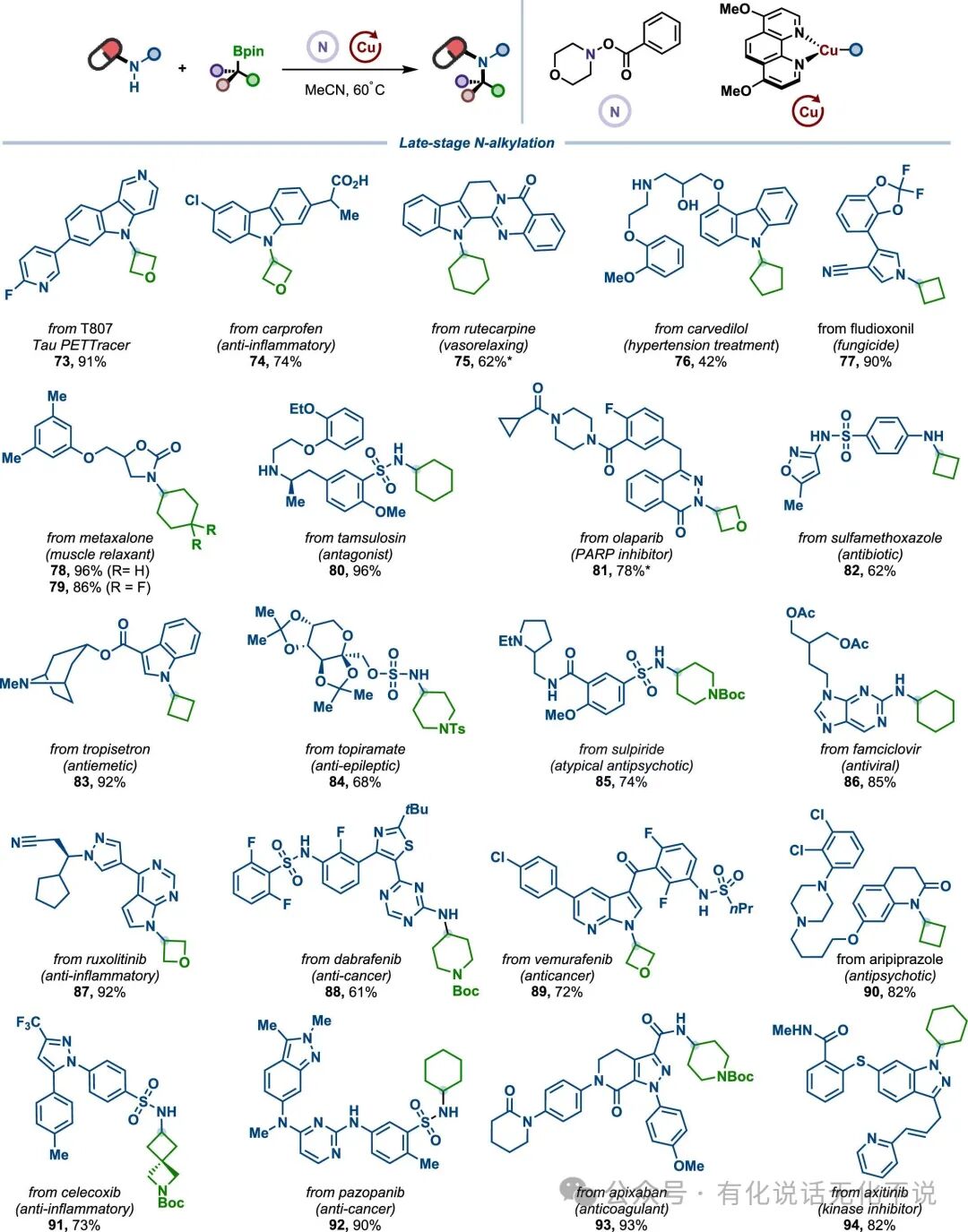
【4】The method is broadly applicable, including but not limited to aromatic amines such as indoles, nitrogen-containing indoles, pyrazoles, imidazoles, pyrroles, and carbazoles, and can also be extended to substrates like sulfonamides, imines, and indole ketones;
【5】The method has also been applied in the late-stage functionalization of complex drug molecules, achieving outstanding results, particularly for substrates 85 & 90, where aliphatic amines are intolerant under photocatalytic conditions, but this method remains unaffected;
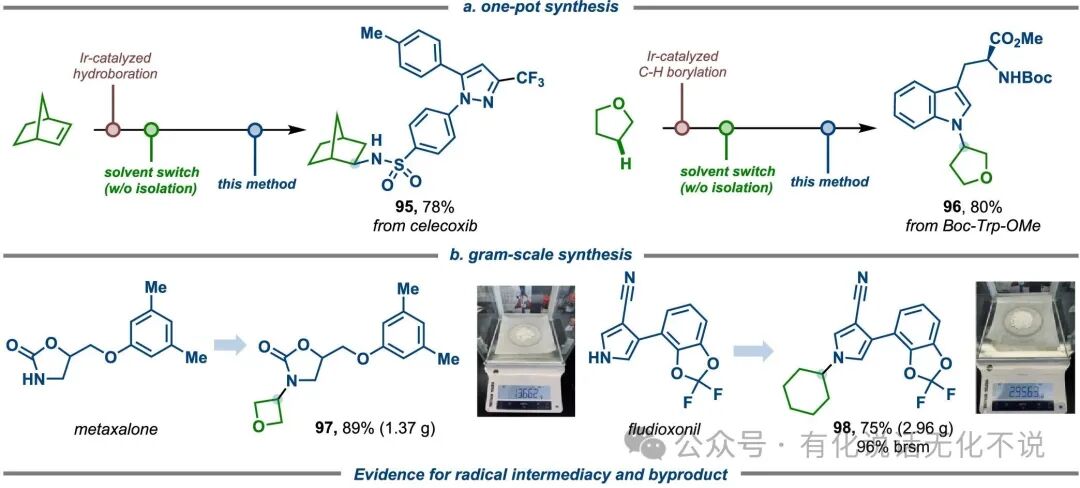
【6】Combining with olefin hydroboration reactions, this method still performs excellently in gram-scale applications of drug molecules;
 【7】Mechanistic studies indicate that the morpholine N• is key to the reaction;
【7】Mechanistic studies indicate that the morpholine N• is key to the reaction;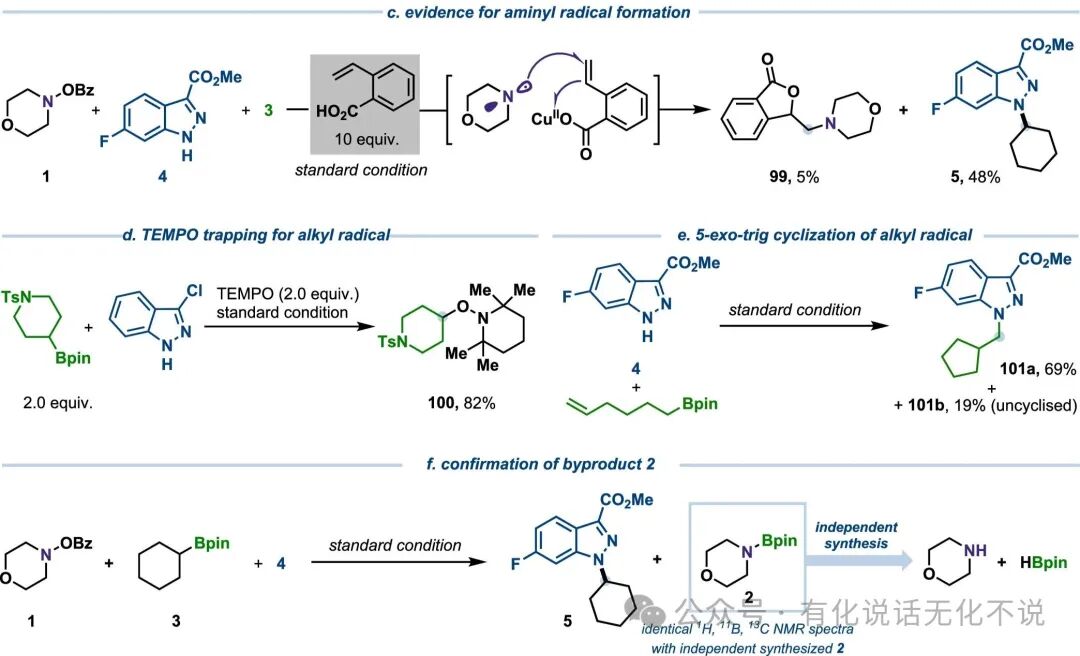
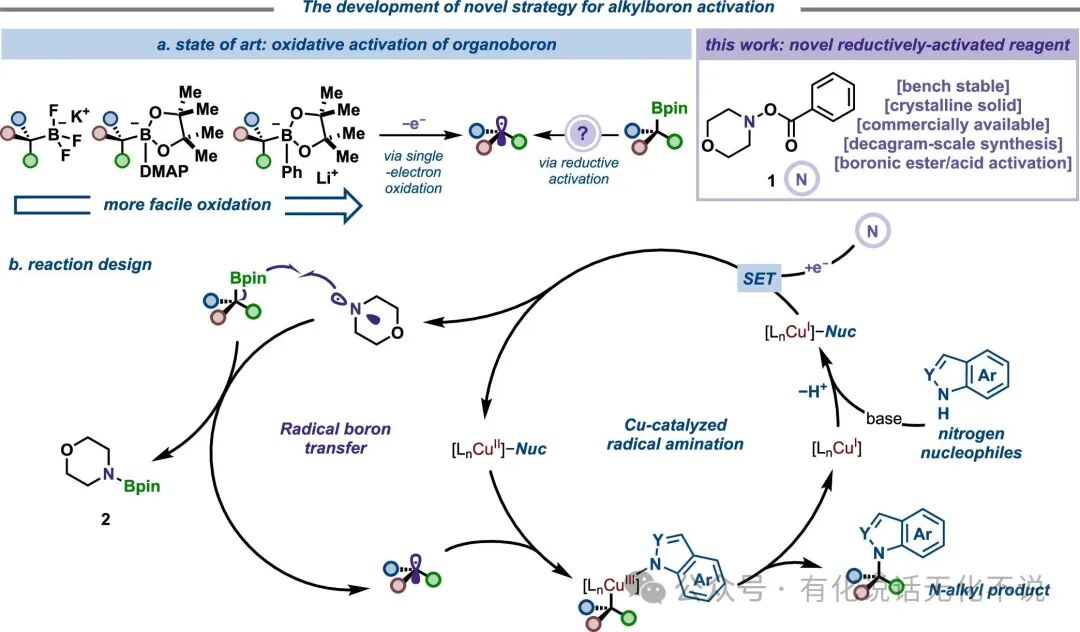 【8】Morpholine benzoic acid ester【CAS#5765-65-1】is a commercially available reagent, easy to obtain.⭐References⭐Radical Strategy to the Boron-to-Copper Transmetalation Problem: N-Alkylation with Alkylboronic EstersRuocheng Sang, Jason E. GestwickiJ. Am. Chem. Soc. 2025, XXXX, XXX, XXX-XXXDOI : 10.1021/jacs.5c07856
【8】Morpholine benzoic acid ester【CAS#5765-65-1】is a commercially available reagent, easy to obtain.⭐References⭐Radical Strategy to the Boron-to-Copper Transmetalation Problem: N-Alkylation with Alkylboronic EstersRuocheng Sang, Jason E. GestwickiJ. Am. Chem. Soc. 2025, XXXX, XXX, XXX-XXXDOI : 10.1021/jacs.5c07856