
 Image source: J. Am. Chem. Soc.Introduction:
Image source: J. Am. Chem. Soc.Introduction:
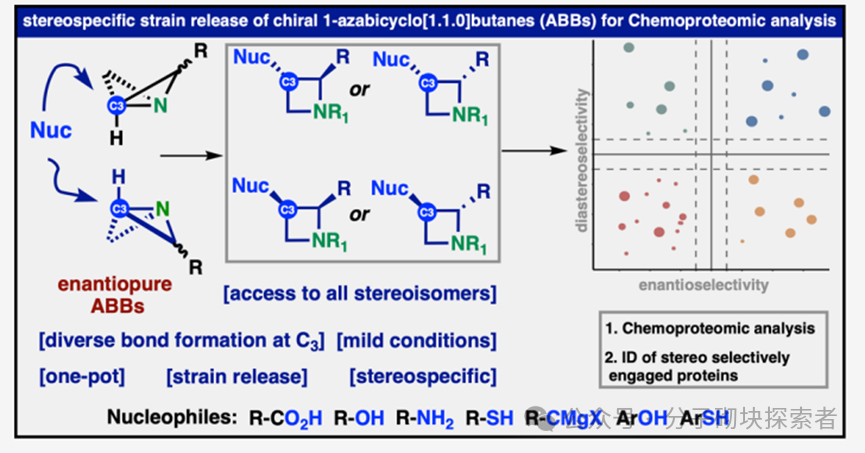
Professors Benjamin F. Cravatt and Phil S. Baran developed a concise, modular, and programmable synthetic strategy based on strain-release functionalization reactions for constructing structurally complex stereopure nitrogen-containing bicyclic compounds known as Azetidine. This synthetic method allows for the parallel preparation of nitrogen-containing bicyclic compounds with defined stereostructures, which is challenging to achieve efficiently using traditional methods. Given the advantageous properties of such structures, a set of stereoprobe reagents for activity-based protein profiling (ABPP) was synthesized, which were found to bind to specific proteins in human cancer cells, exhibiting significant stereoselectivity and chemical selectivity.

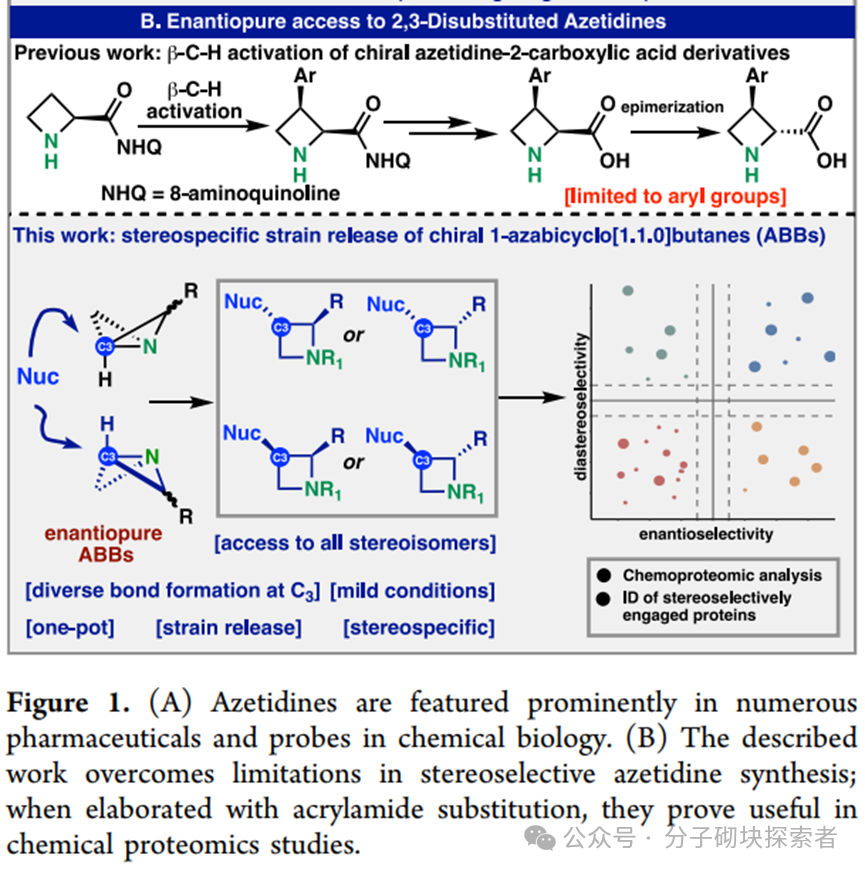 Image source: J. Am. Chem. Soc.
Image source: J. Am. Chem. Soc.
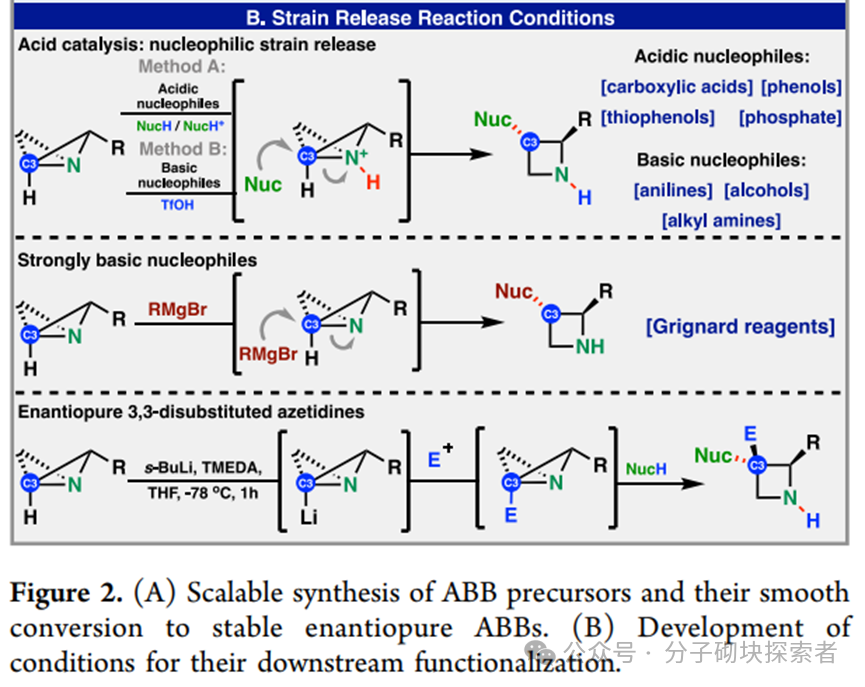 Image source: J. Am. Chem. Soc.
Image source: J. Am. Chem. Soc.
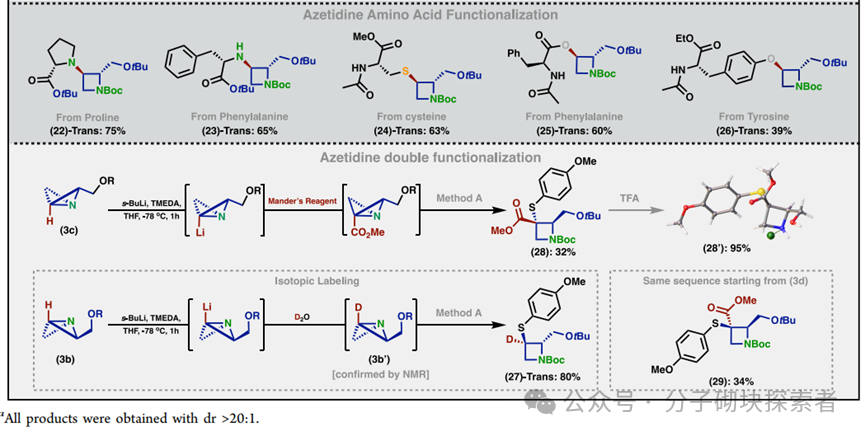 Image source: J. Am. Chem. Soc.
Image source: J. Am. Chem. Soc.



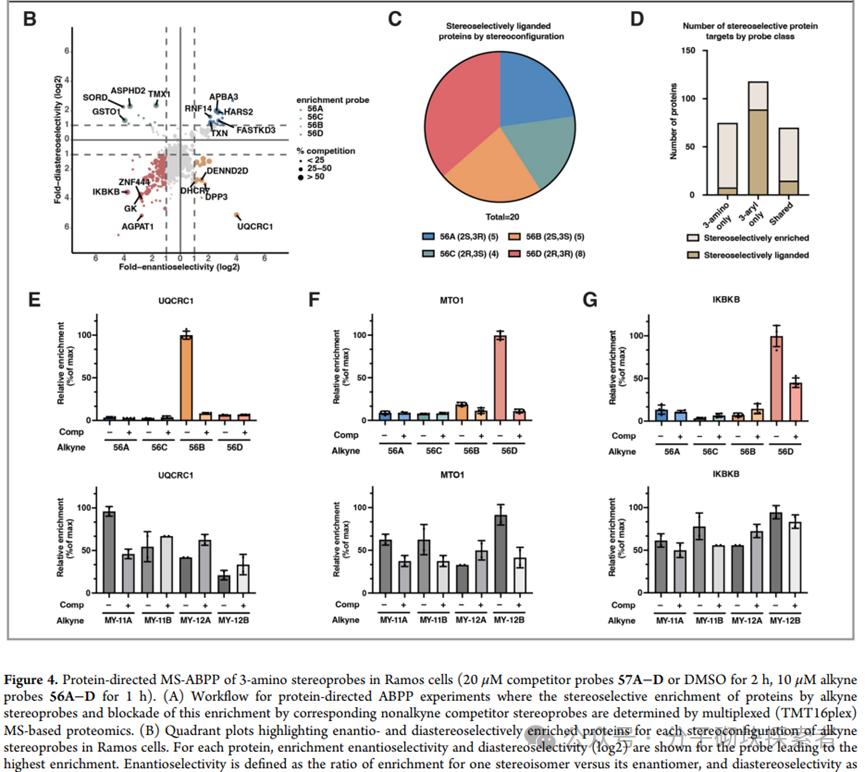 Image source: J. Am. Chem. Soc.Conclusion:
Image source: J. Am. Chem. Soc.Conclusion:
A modular synthetic strategy has been reported that enables the rapid acquisition of enantiopure substituted nitrogen-containing bicyclic compounds with either cis or trans configurations. The approach described in this paper allows for the introduction of diverse functional group patterns and the construction of products with fully substituted C-3 stereocenters. Various nitrogen-containing bicyclic derivatives were synthesized, along with a complete set of acrylamide stereoprobes for chemical proteomics research—these studies revealed differential interactions of 3-aryl and 3-amino stereoprobes with proteins in human cancer cells. Subsequent research could further expand the structural complexity of accessible nitrogen-containing bicyclic compounds (e.g., achieving higher-order substitutions) and utilize cysteine-directed activity-based protein profiling (ABPP) techniques to locate cysteine binding sites on targets of 3-amino stereoprobes. Notably, it was found that 3-aryl stereoprobes can: stereoselectively disrupt the regulatory proteasome complex 4c, inhibit RNA methyltransferase 4d, and serve as E3 ligase ligands for targeted protein degradation 4a. Based on these findings, the functional impacts of the newly discovered interactions of 3-amino stereoprobes will be emphasized. It is particularly noted that these functional studies can employ various chemical controls (inactive enantiomer stereoprobes; stereoprobes lacking electrophilic acrylamide) and biological controls (stereoprobe-resistant cysteine mutant proteins) to verify whether the observed biological effects are target-specific.
References:
Enantiocontrolled Azetidine Library Synthesis via Strain-Release Functionalization of 1-Azabicyclobutanes
J. Am. Chem. Soc. 2025, 10.1021/jacs.5c07227
https://doi.org/10.1021/jacs.5c07227