
Soy Protein Isolate (SPI) is an important plant protein that is widely used in the food industry, agriculture, and biotechnology due to its high nutritional value, low production cost, excellent biocompatibility, and processing characteristics. Numerous studies have shown that the functional properties of SPI can be improved through non-covalent or covalent binding with small molecular active substances such as polyphenols, thereby enhancing its application value.

Ultrasonic treatment can change the molecular structure of proteins to some extent, thereby affecting their functional properties, and is a common physical treatment method. While there has been research on ultrasound and polyphenol non-covalent/covalent modification of proteins, there are few reports on the combined modification of proteins by ultrasound and polyphenols. In this experiment, to explore the effects of ultrasonic combined with polyphenol modification on protein structure and function, Dai Shicheng, Jiang Lianzhou*, Wang Huan*, and others from the College of Food Science, Northeast Agricultural University, treated SPI with ultrasound, and under the conditions of pH 3, 7, 9, and 12, prepared SPI-catechin non-covalent/covalent complexes through non-covalent/covalent interactions. They better analyzed the interactions between SPI and catechin and the structural changes through fluorescence spectroscopy, infrared spectroscopy, SDS-PAGE, and molecular docking technology, and compared the effects of combined treatment on functional properties such as solubility, foaming ability, and antioxidant activity. The research results can provide a theoretical basis and research evidence for the application of ultrasonic combined acid/base treatment in the field of protein modification and the use of soybean protein-polyphenol complexes as functional food ingredients.

Effect of Ultrasound on SPI Structure
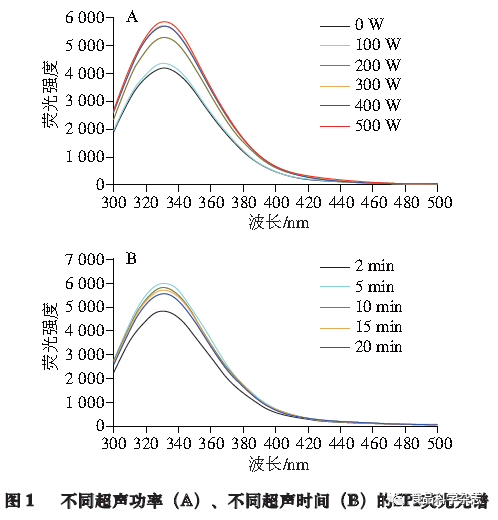
As shown in Figure 1A, when the ultrasonic time is fixed at 5 min, the fluorescence intensity of SPI significantly increases after treatment with different ultrasonic powers, with the maximum fluorescence intensity increase occurring at an ultrasonic power of 500 W. As shown in Figure 1B, when the ultrasonic time is fixed at 5 min, after treatment with different ultrasonic powers, the fluorescence intensity of SPI significantly increases, with the maximum increase occurring at an ultrasonic power of 500 W. When the ultrasonic power is fixed at 500 W, and different ultrasonic treatment times are applied, it was found that under the condition of 5 min, the fluorescence intensity increases the most, indicating that under the ultrasonic treatment conditions of 500 W and 5 min, the structure of SPI is opened to the greatest extent, exposing the tryptophan residues the most. This also indicates that ultrasonic treatment can promote the opening of the internal hydrophobic regions of the protein, which may facilitate binding with catechin.
Effect of pH on the Secondary Structure of Protein-Catechin Complexes
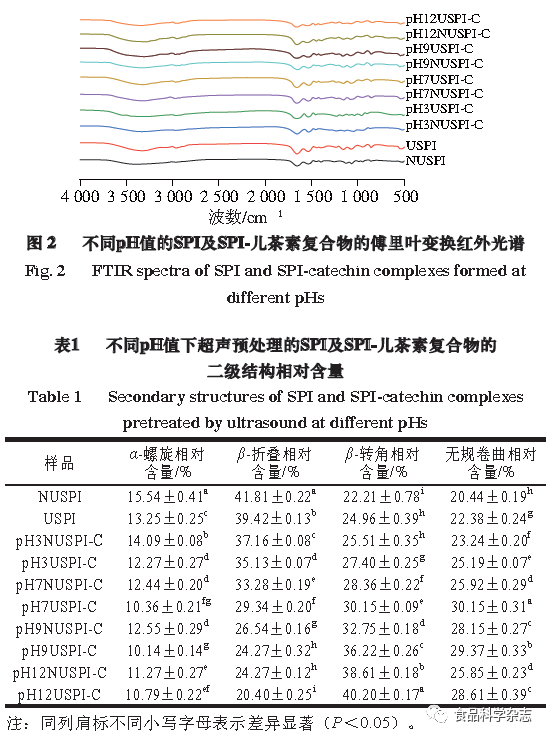
Figure 2 shows the infrared spectra of SPI pretreated with ultrasound forming complexes with catechin at different pH values. After treatment under different conditions, the amide I band (1600~1700 cm-1) shows significant changes, mainly attributed to the C=O stretching vibration, N—H bending, and C—H stretching vibrations. The absorption peak of the amide I band shifts to lower frequencies, which may be due to the formation of hydrogen bonds through intramolecular associations of the protein after ultrasonic treatment. The secondary structure content of the sample infrared spectra fitting is shown in Table 1. Compared with NUSPI, the content of α-helix and β-sheet significantly decreases after ultrasonic treatment, while the content of β-turn and random coil significantly increases (P<0.05), indicating that ultrasound makes the structure of SPI more loose and disordered. Along with the addition of catechin and the change of pH, a similar trend is observed in the resulting complexes, with a large amount of α-helix and β-sheet converting into β-turn and random coil, especially under conditions of pH 12 and ultrasonic treatment (P<0.05), which makes the SPI structure more loose and stretched, exposing the hydrophobic and polar groups of the protein peptide chain to the surface, increasing the interaction degree between SPI and catechin.
Effect of pH on Protein-Catechin Interactions
As shown in Figure 3, compared to NUSPI, the possible reason for the increase in fluorescence intensity is that the cavitation effect produced by ultrasound changes the internal polar microenvironment of the protein molecules, making the protein structure loose, exposing the tryptophan residues inside, which leads to an increase in fluorescence intensity. As catechin is added and pH increases, the degree of fluorescence intensity decrease of the complexes gradually increases. Compared to the NUSPI-C group, the fluorescence intensity decrease of the USPI-C complex is greater, and the decrease in fluorescence intensity under covalent bonding is more significant, with the USPI-C complex at pH 12 showing the most significant decrease, with a reduction of 89.16% compared to NUSPI, and a significant red shift (5 nm), which may be due to the ultrasonic treatment opening the internal hydrophobic regions of SPI, making the structure loose, and the irreversible covalent bonding of catechin with the protein under alkaline conditions greatly reduces the tryptophan content. The side chain amine (—NH2) of tryptophan forms covalent cross-links with catechin, generating C—N covalent bonds, which masks some tryptophan residues, altering the hydrophobic environment around tryptophan and reducing fluorescence intensity.

Effect of pH on the Molecular Weight of Protein-Catechin Complexes
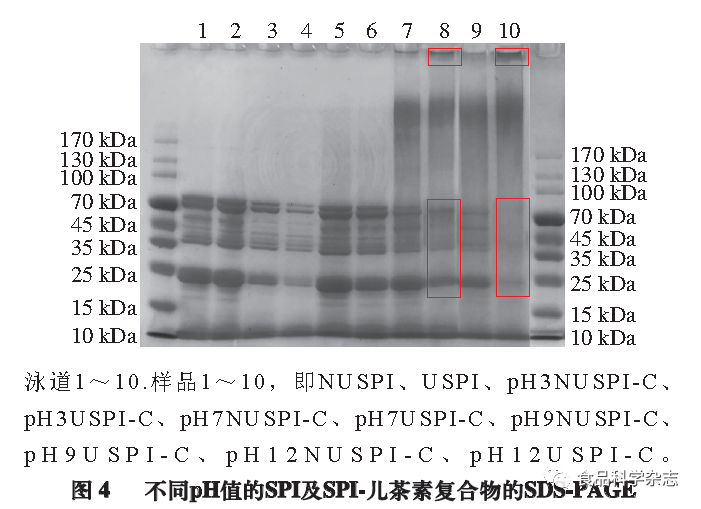
As shown in Figure 4, there is no significant difference between samples 1 and 2, indicating that the 500 W ultrasound has little effect on the subunit composition of SPI. The appearance of black bands at the top of the separation gel for samples 7 to 10 indicates the formation of new complexes, with samples 8 and 10 being USPI-C complexes, where the top band is darker than that of samples 7 and 9 (NUSPI-C), possibly because the internal structure of SPI opened after ultrasound treatment, increasing binding sites and promoting binding with catechin. Sample 10 may have formed stronger covalent cross-links due to a greater degree of structural opening after ultrasound and alkaline treatment, confirming that covalent complexes are formed between SPI and catechin. Samples 3 and 4 may have lighter electrophoresis bands due to lower soluble protein concentrations prepared at pH 3, and samples 3 to 6 did not form new bands, indicating that SPI and catechin did not undergo covalent cross-linking at pH 3 and pH 7, and did not change the quaternary structure of the protein.
Effect of pH on the Solubility of Protein-Catechin Complexes
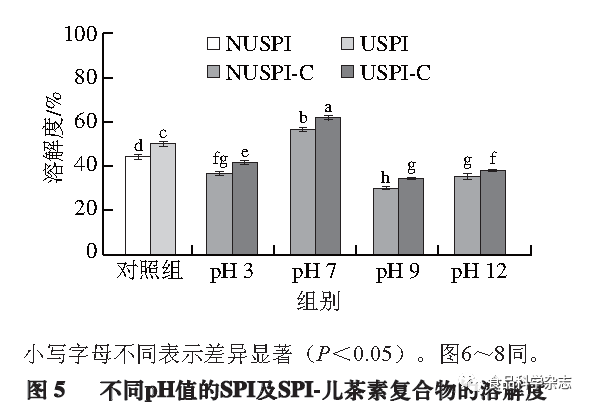
The solubility of proteins is an important functional characteristic in food applications, as insoluble proteins may limit the expression of other functional properties. Its solubility depends on certain intrinsic factors (such as protein amino acid composition and sequence) and extrinsic factors (pH, temperature, ionic strength). As shown in Figure 5, the solubility of SPI significantly increases after ultrasonic treatment (P<0.05), possibly because ultrasound causes SPI to break down into smaller particles, increasing the specific surface area of the protein and promoting interactions between the protein and water, leading to increased solubility. The solubility of the complexes formed between SPI and catechin at different pH values varies significantly; at pH 3, the solubility may decrease due to the proximity of the pH of the solution system to the isoelectric point of SPI, leading to some protein precipitation. At pH 7, the solubility increases compared to pure protein, possibly because the hydroxyl (-OH) groups introduced by catechin form hydrogen bonds with water molecules, enhancing the hydration of the complexes. However, at pH 9 and pH 12, the addition of catechin significantly reduces protein solubility (P<0.05), possibly due to catechin oxidizing into theaflavins (quinone compounds) under alkaline conditions, forming C-N bonds with SPI side chains to create a large, insoluble complex, and the molecular weight also increases. The SDS-PAGE results further confirm this conclusion. As shown in Figure 5, for the complexes at the same pH, ultrasonic pretreatment increases their solubility, likely because the structure of SPI after ultrasonic treatment opens more, binding more catechin while also introducing a large number of hydrophilic groups (hydroxyl and carboxyl), thus increasing solubility.
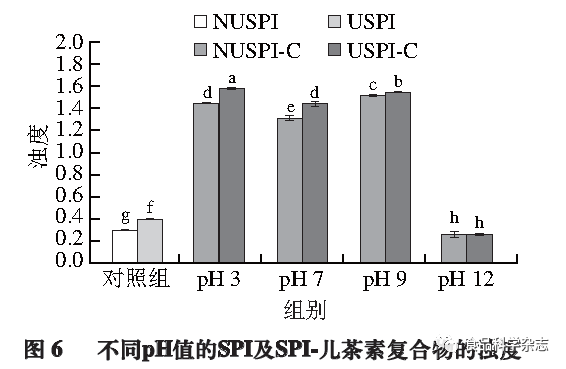
Effect of pH on the Turbidity of Protein-Catechin Complexes
Turbidity analysis is an intuitive method for detecting the interactions between proteins and polyphenols, where an increase in turbidity can be attributed to the increase in the number and size of complex aggregates. As shown in Figure 6, after the introduction of catechin, the turbidity of the complexes formed at pH 3, 7, and 9 significantly increases compared to SPI (P<0.05), indicating the formation of larger particles in the solution. At pH 3 and pH 7, non-covalent complexes are formed, with the turbidity of the pH 3 complex being higher than that of pH 7, possibly due to the proximity of the pH to the isoelectric point of SPI causing protein precipitation. At pH 9 and pH 12, covalent complexes are formed, while the turbidity of the pH 12 complex is significantly lower than that of pH 9 (P<0.05), possibly because pH 12 is further from the isoelectric point of SPI. The higher turbidity at pH 9 may be due to the covalent cross-linking of SPI with catechin under alkaline conditions forming larger network complexes. Ultrasonic pretreatment clearly increases the turbidity of the solution, possibly because the internal structure of SPI opens after ultrasound, binding more catechin to form larger molecular weight aggregates, which is also confirmed by electrophoresis results.
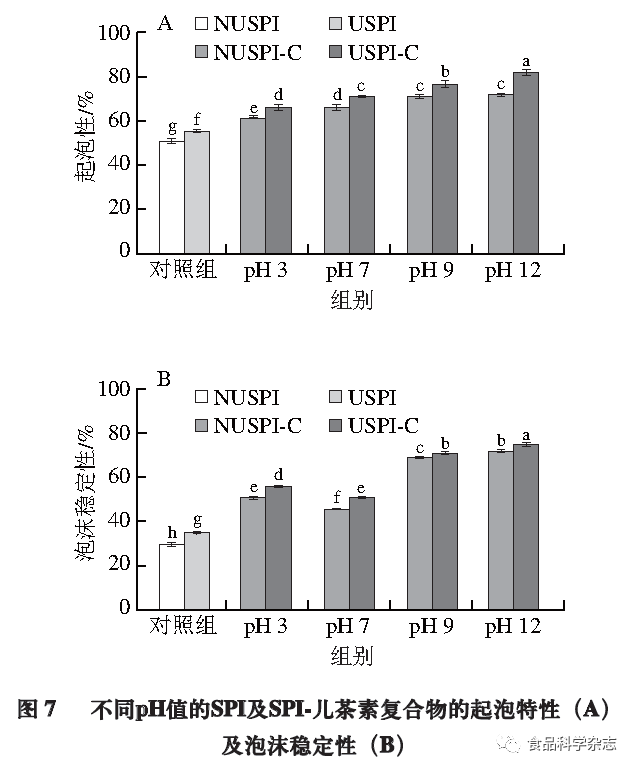
Effect of pH on the Foaming Properties of Protein-Catechin Complexes
Foaming properties are crucial for the quality of many foods, such as milk, ice cream, whipped cream, cakes, and bread. As shown in Figure 7, with the addition of catechin and the increase of pH, the foaming ability and stability of SPI significantly enhance (P<0.05). The protein-catechin complexes exhibit higher inherent viscosity, producing more stable foams, possibly because the addition of catechin reduces the surface tension at the SPI interface, allowing the protein structure to unfold and interact with it, making the interfacial film more stable. The addition of catechin under alkaline conditions has a stronger effect on protein foaming than under acidic conditions, possibly because the covalent cross-linked structure can effectively enhance the protein’s expansion at the air/liquid interface, forming more foam network structures, which enhances foam formation by inhibiting protein aggregation and disproportionation, thus improving foaming ability. Moreover, the covalent complex formed under alkaline conditions between SPI and catechin through covalent bonds with a network structure is more stable, resulting in better foaming stability. The protein-catechin complexes formed after ultrasonic treatment of SPI exhibit significantly better foaming ability and stability than those formed from untreated proteins, likely because the ultrasound-treated SPI dissolves more and binds more catechin, which is beneficial for enhancing foaming performance.
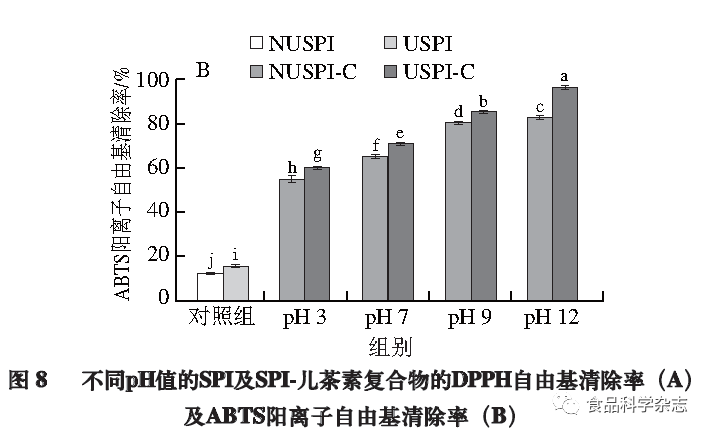
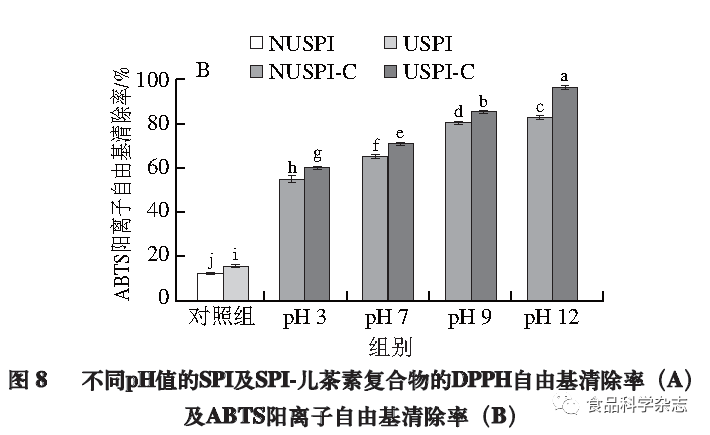
Effect of pH on the Antioxidant Activity of Protein-Catechin Complexes
This experiment explores the effect of ultrasonic pretreatment on the antioxidant capacity of SPI-catechin complexes at different pH values through DPPH and ABTS radical scavenging abilities. As shown in Figure 8, the DPPH radical scavenging rate and ABTS radical scavenging rate trends are basically consistent; with the increase of pH and SPI ultrasonic pretreatment, the antioxidant capacity of the formed complexes gradually enhances, with the most significant increase at pH 12 (P<0.05), where the DPPH radical scavenging rate increases to 5.5 times that of SPI, and the ABTS radical scavenging rate increases to 4.8 times that of SPI. This may be due to ultrasonic pretreatment opening the internal structure of SPI, exposing more hydrophobic groups to bind with catechin, while catechin introduces a large number of hydrogen ions, reacting with DPPH to generate DPPH·H, and ABTS generates a large number of cationic free radicals after oxidation, enhancing antioxidant capacity. The antioxidant activity at pH 3 is poorer, possibly because the proximity of pH 3 to the isoelectric point of SPI leads to partial precipitation of the complexes, which prevents them from exerting their antioxidant performance. However, at pH values of 7, 9, and 12, the number of covalently bonded catechins under alkaline conditions is greater than that of non-covalently bonded catechins, thus the antioxidant capacity of the covalent complexes is stronger.
Molecular Docking Analysis Results
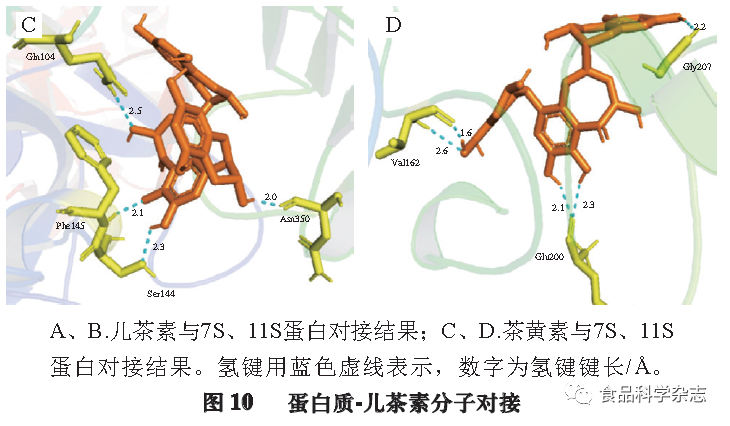
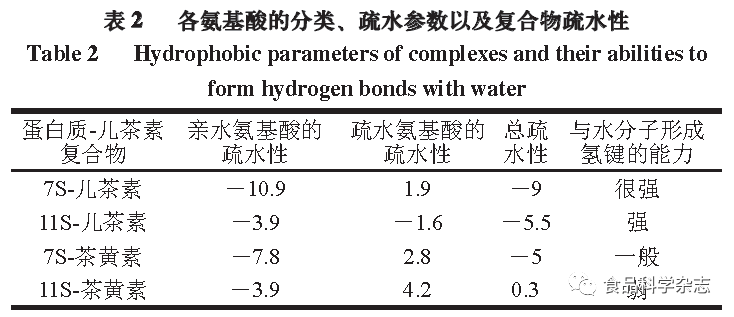
According to sedimentation coefficients, SPI can be divided into four components: 2S, 7S, 11S, and 15S proteins, with 7S and 11S proteins being the main components of SPI, accounting for about 70% of SPI. The oxidation products of catechin are theaflavins (see Figure 9), thus molecular docking simulations were conducted to investigate the hydrogen bonding and hydrophobic interactions involving these two proteins and catechin with theaflavins. As shown in Figure 10, catechin interacts primarily through hydrogen bonds with the Gly88, Gln354, Gln77, Met353, and Glu358 residues of the 7S protein. Catechin mainly interacts with the Glu172, Gly202, and Pro160 residues of the 11S protein through hydrogen bonds. Theaflavins primarily interact with the Gln104, Phe145, Ser144, and Asn350 residues of the 7S protein through hydrogen bonds, and with the Gly207, Val162, and Glu200 residues of the 11S protein through hydrogen bonds. Several hydrophobic amino acid residues within the protein molecules provide significant hydrophobic interactions for the protein-polyphenol complexes, and hydrophobic interactions play a key role in the formation of protein-polyphenol complexes. Table 2 compares the total hydrophobicity of non-covalent complexes to covalent complexes calculated by the hydrophobic parameters of each amino acid, indicating that non-covalent complexes have stronger hydrogen bonding and hydrophobic interaction capabilities than covalent complexes, promoting the hydrophilicity of the complexes, which can enhance other physicochemical properties and biological activity by increasing protein solubility, further validating that the main forces in non-covalent complexes are hydrogen bonding and hydrophobic interactions.
This experiment explored the effects of ultrasonic pretreatment and pH on the structure and function of SPI-catechin non-covalent/covalent complexes. Under optimized conditions of 5 min ultrasonic time and 500 W power, SPI and catechin formed complexes through non-covalent/covalent bonding. Through Fourier transform infrared spectroscopy, fluorescence spectroscopy, and SDS-PAGE results, it was found that the interaction between SPI and catechin is strongest at pH 12 after ultrasonic pretreatment, with significant reductions in α-helix and β-sheet content of SPI, and significant increases in β-turn and random coil content. Additionally, both formed high molecular weight covalent polymers. Furthermore, the foaming ability, foam stability, and antioxidant capacity of the SPI-catechin complexes at pH 12 were significantly stronger than those at pH 3, 7, and 9. Molecular docking results indicated that non-covalent bonding more easily generates hydrogen bonds and hydrophobic interactions than covalent bonding. The above experimental results can provide theoretical guidance for the application of protein-catechin complexes in functional food ingredients and the field of protein ultrasonic modification.
Dai Shicheng, Male, Master’s student of the 19th cohort at the College of Food Science, Northeast Agricultural University, research direction in grain, oil, and plant protein engineering.

Jiang Lianzhou, Professor, Doctoral Supervisor, former Dean of the College of Food Science at Northeast Agricultural University, Director of the National Soybean Engineering Technology Research Center. Also serves as a member of the State Council Academic Degrees Committee, Soybean Industry System Scientist, member of the Ministry of Education Science and Technology Committee, Chairman of the China Section of the American Oil Chemists’ Society (AOCS), Vice President of the China Soybean Industry Association, etc. He is a valid candidate for the Chinese Academy of Engineering in 2019 and was elected as a fellow of the International Union of Food Science and Technology (IUFoST) in 2020. He has hosted nearly 50 major national research projects such as UNDP and the “863 Program”, published 220 papers in SCI/EI, published 11 books including national-level planning textbooks for the “Twelfth Five-Year Plan”; received more than 30 provincial and ministerial-level scientific and technological awards; holds nearly 100 authorized invention patents; transformed over 40 scientific and technological achievements, creating economic benefits of 10 billion yuan for enterprises; trained over 150 doctoral and master’s students; as the person in charge, established the National Soybean Engineering Center, Soybean Industry Technology Innovation Strategic Alliance, etc.; submitted more than 20 research reports and decision-making suggestions to relevant departments of the State Council and local governments, contributing significantly and playing a leading role in the discipline and industry.

Wang Huan, Lecturer, Master’s Supervisor, Teacher at the College of Food Science, Northeast Agricultural University, has been engaged in research on grain, oil, and plant protein since 2011. He has hosted 6 projects/topics, including the “Excellent Youth” fund project of the Heilongjiang Provincial Natural Science Foundation, the China Postdoctoral Fund project, and the Heilongjiang Provincial Postdoctoral Fund project; participated in 17 national and provincial-level projects; publicly published 40 papers, of which 32 are SCI/EI; published 7 books (including 1 as co-editor, and 2 as contributors to national-level planning textbooks for the Twelfth and Thirteenth Five-Year Plans); holds 9 authorized patents, has 29 accepted patents, and has applied for 1 international invention patent; received 14 provincial and ministerial-level awards including the First Prize of Technological Invention from the China Light Industry Federation, and the First Prize of Science and Technology Award from the China Food Industry Association; participated in a total of 20 domestic and international academic conferences and reports; assisted in hosting 8 important international and domestic academic conferences.
This article “Effects of Ultrasonic Pretreatment on Soy Protein-Isolate-Catechin Non-Covalent/Covalent Complex Structure and Function” is from “Food Science”, 2022, Vol. 43, Issue 1, Pages 102-110, Authors: Dai Shicheng, Lian Zitong, Ma Linzhi, Tong Xiaohong, Tian Tian, Qi Weijie, Peng Chaoyong, Fan Yuhang, Wang Huan*, Jiang Lianzhou* DOI: 10.7506/spkx1002-6630-20210202-031. Click below to read the original article.
“Food Science”: The Effects of Different Antioxidants on the Oxidative Stability of Meat Products
“Food Science”: Research Progress on Non-Destructive Detection Technology of Citrus Near-Infrared Spectroscopy
“Food Science”: The Isolation and Purification of Polysaccharides from Asparagus and Their In Vitro Antioxidant Activity
“Food Science”: Research and Application Progress of Lactic Acid Bacteria in Antagonizing Foodborne Pathogens
“Food Science”: Research Progress on the Preparation and Application of Natural Polysaccharide Microgels
“Food Science”: The Effects of Ethephon Treatment on the Phenolic Composition of Cabernet Sauvignon Grapes in Wine
“Food Science”: The Construction, Functional Properties, and Applications of Metal-Polyphenol Networks in the Food Field
“Food Science”: The Mechanism of Tryptophan Inhibition of Late Glycation End Product Formation in In Vitro Models
“Food Science”: The Application of Colloidal Structure Design in Reducing Salt Foods
“Food Science” Expert Invitation: Research Progress on the Chemical Composition and Biological Activity of Polysaccharides in Flowers
“Food Science”: The Preparation of Tomatine and Its Inhibitory Effect on Acetylcholinesterase
“Food Science”: Quick Detection of 5′-Inosine Monophosphate Content in Soup Based on Citric Acid-Europium Nano Coordination Polymer Fluorescent Probe
“Food Science”: Analysis and Comprehensive Evaluation of Amino Acid Composition of Different Varieties of Almonds
“Food Science”: The Effects of Fermentation on the Quality Characteristics of Black Pig Jerky
“Food Science”: Analysis of Volatile Organic Compound Fingerprints of Ginger from Different Origins Based on Gas Phase Ion Mobility Spectrometry
“Food Science”: Quality Analysis and Comprehensive Evaluation of Australian Macadamia Nuts
“Food Science”: High-Throughput Sequencing Analysis of Vomitoxin-Degrading Bacteria in Mixed Microbial Communities
“Food Science”: The Effects of Monascus on the Physicochemical and Rheological Properties of White Mold Cheese
Modified by: Northeast Agricultural University, College of Food Science, Dai Shicheng; Editor/Responsible Editor: Zhang Ruimei
Images sourced from the original article.





















