Soy protein fibers are a new type of soy protein produced using unmodified defatted soybean meal and soy protein isolate (SPI) through twin-screw extrusion technology, commonly used as a meat substitute. During the extrusion process, various complex processing operations can lead to changes in the structure and physicochemical properties of food polymers.
Professors Zhang Haojia, Zhu Xiuqing*, and Sun Ying from the School of Food Engineering at Harbin University of Commerce systematically studied the effects of extruder barrel temperature, screw speed, and material moisture content on the solubility, particle size, and secondary structure of soy protein, to explore the conformational changes of soy protein during the extrusion process, aiming to clarify the mechanism of extrudate formation and provide a theoretical basis for the quality optimization of soy protein fibers.

01
Results and Analysis
1.1.2 Impact of Different Material Moisture Contents on SPI Nitrogen Solubility Index
1.2.1 Impact of Different Barrel Temperatures on SPI Free Sulfhydryl and Disulfide Bond Content
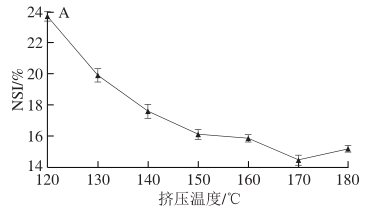
As shown in Figure 2A, with the increase in extrusion temperature, the content of disulfide bonds in SPI first increases and then decreases, while the content of free sulfhydryl groups shows a trend of first decreasing and then stabilizing. This is presumed to be because after SPI undergoes extrusion treatment, its natural structure is destroyed, molecular chains are opened, and intramolecular sulfhydryl groups are exposed. Additionally, some free sulfhydryl groups are converted into disulfide bonds, leading to insignificant changes in free sulfhydryl content. It can be inferred that disulfide bonds play an important role in the formation of SPI aggregates; when the temperature exceeds 160 °C, the content of disulfide bonds slightly decreases, indicating that excessively high temperatures are not conducive to the exposure of intramolecular sulfhydryl groups and the formation of disulfide bonds. Relevant studies also indicate that excessively high extrusion temperatures can destroy the molecular bonds formed directly by protein molecules.
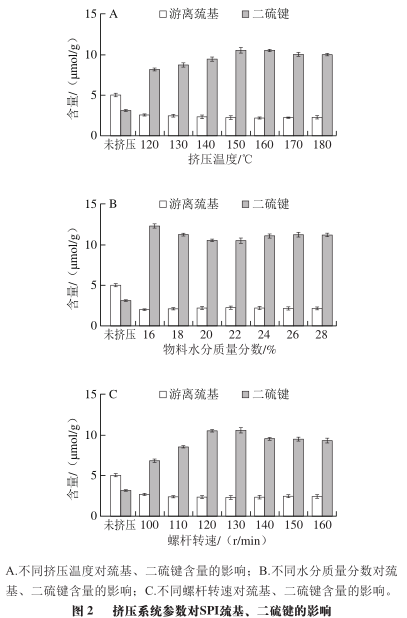
1.2.2 Impact of Different Material Moisture Contents on SPI Free Sulfhydryl and Disulfide Bond Content
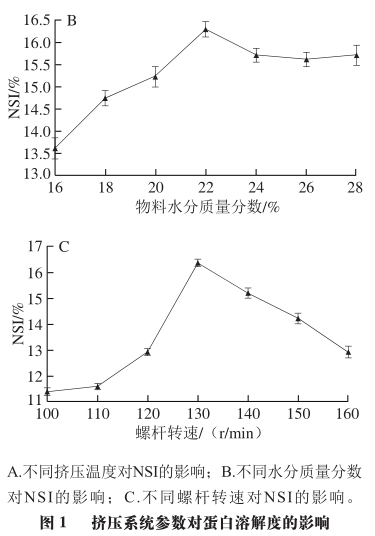
As shown in Figure 2B, as the material moisture content increases from 16% to 22%, the content of disulfide bonds gradually decreases, while the change in the content of free sulfhydryl groups is not significant. This indicates that under these moisture conditions, the material, due to the protective lubrication effect of water, is not conducive to the exposure of internal sulfhydryl groups and the formation of disulfide bonds. Both lower or higher material moisture contents exacerbate the damage to the natural conformation of SPI during extrusion treatment; at a material moisture content of 22%, the content of disulfide bonds is at its lowest, resulting in the highest solubility of extruded SPI.
1.2.3 Impact of Different Screw Speeds on SPI Free Sulfhydryl and Disulfide Bond Content
As shown in Figure 2C, with the increase in screw speed, the content of free sulfhydryl groups decreases while the content of disulfide bonds shows a trend of first increasing and then decreasing. This indicates that after SPI undergoes the shear force of the screw, the natural structure of the protein is damaged, and the internal groups are exposed, leading to an increase in disulfide bond content. The highest content of disulfide bonds is reached at a screw speed of 130 r/min, at which point SPI aggregates to a higher degree under the influence of disulfide bonds and has higher solubility. However, when the screw speed exceeds 130 r/min, the content of disulfide bonds slightly decreases, indicating that excessively high screw speeds are not conducive to the exposure of internal sulfhydryl groups and the formation of disulfide bonds, further reducing the solubility of SPI and decreasing the proportion of low molecular weight components in SPI subunits.
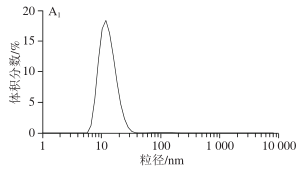
1.3.1 Impact of Different Barrel Temperatures on SPI Particle Size Volume Distribution
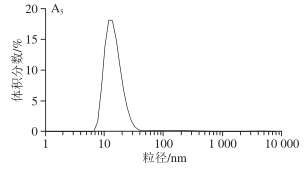
In its natural state, SPI samples exhibit a unimodal distribution, and the particle size distribution is relatively uniform. After extrusion treatment, the SPI particle size volume distribution graph changes from unimodal to bimodal or trimodal. With the increase in extrusion temperature, the particle size distribution gradually shifts to the right, and the average particle size of SPI shows an increasing trend. Larger protein particle sizes will reduce their solubility. As shown in Table 2, the average particle size of SPI increases to its maximum value at 150 °C, and a larger absorption peak for particle size appears in the spectrum in Figure 3A, presumed to be due to the fact that as the temperature increases, the protein molecular chains depolymerize and re-aggregate into larger aggregates, leading to an increase in average particle size. However, when the temperature continues to increase beyond 160 °C, the larger absorption peaks gradually disappear, but the distribution remains broad, indicating that high-temperature extrusion conditions will destroy some of the larger SPI aggregates. This phenomenon may also be due to the fact that the loose structure formed by SPI after high-temperature treatment is easily damaged.
|
|
|
|
|
|
|
|
|

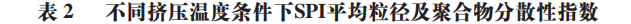
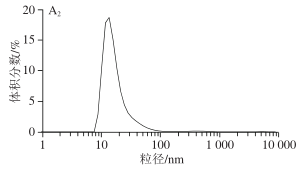
1.3.2 Impact of Different Material Moisture Contents on SPI Particle Size Volume Distribution


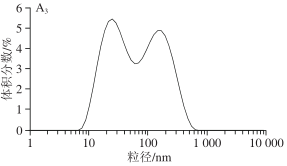
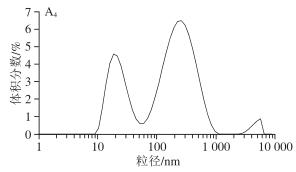
1.3.3 Impact of Different Screw Speeds on SPI Particle Size Volume Distribution


1.4.1 Impact of Different Barrel Temperatures on SPI Zeta Potential
The absolute value of Zeta potential indicates the amount of positive or negative charges on the molecular surface. The higher the absolute value of SPI Zeta potential, the more charges on the protein molecular surface, and under greater electrostatic repulsion, SPI structure can maintain a relatively stable state. Compared to unextruded SPI, extrusion treatment significantly increases the absolute value of SPI Zeta potential; as shown in Figure 4A, with the increase in extrusion temperature, the absolute value of SPI Zeta potential first increases and then decreases. At an extrusion temperature of 160 °C, the absolute value of SPI Zeta potential reaches its highest point, indicating that the electrostatic repulsion between SPI molecules is maximized. This shows that after extrusion treatment, the number of protein aggregates formed through disulfide bonds, hydrophobic interactions, and electrostatic interactions increases. As the extrusion temperature further rises, the absolute value of Zeta potential no longer continues to increase, and the electrostatic charge density on the surface of SPI molecules decreases, indicating that excessively high extrusion temperatures are not conducive to the formation of protein aggregates. Extrusion treatment can destroy the natural structure of SPI while promoting the exposure of internal hydrophobic residues and charged groups, resulting in an increase in the absolute value of SPI Zeta potential.
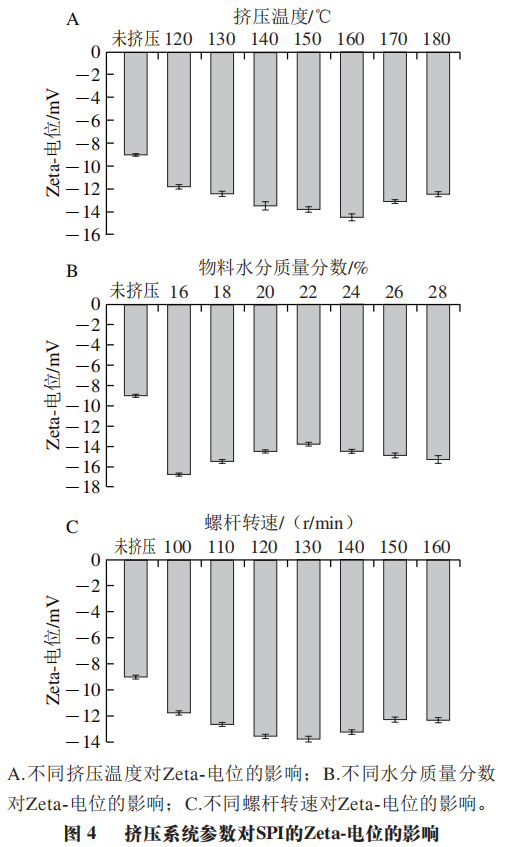
1.4.2 Impact of Different Material Moisture Contents on SPI Zeta Potential
After extrusion treatment, the absolute value of the Zeta potential of the extrudate significantly increases. As shown in Figure 4B, with the increase in material moisture content, the trend shows first a decrease and then an increase. At a material moisture content of 22%, the absolute value of SPI Zeta potential reaches its lowest point, indicating that the electrostatic repulsion between SPI molecules is minimal. This shows that under these conditions, the protein aggregates formed after extrusion treatment are fewer; both higher and lower material moisture contents lead to an increase in the absolute value of Zeta potential, indicating that under conditions of higher or lower material moisture contents, it is beneficial for the formation of insoluble protein aggregates. Combining with the particle size analysis data, both higher and lower material moisture contents lead to lower average particle sizes of the extrudate and higher PDI, making it impossible to form a uniform SPI aggregate system. In summary, the impact of different moisture contents during extrusion treatment on SPI aggregation is significant; lower material moisture contents facilitate the formation of SPI aggregates but cannot form a uniform aggregation system, and higher moisture contents can also disrupt the formation of stable aggregates due to the vaporization of water molecules.
1.4.3 Impact of Different Screw Speeds on SPI Zeta Potential
With the increase in screw speed, the absolute value of SPI Zeta potential first increases and then decreases. As shown in Figure 4C, at a screw speed of 130 r/min, the absolute value of SPI Zeta potential reaches its highest point, indicating that at this point, the electrostatic repulsion between SPI molecules is maximized. Existing studies also indicate that after extrusion treatment, the number of protein aggregates formed through disulfide bonds, hydrophobic interactions, and electrostatic interactions increases. However, as the screw speed further increases, the absolute value of Zeta potential begins to decrease, indicating that excessively high screw speeds input higher mechanical energy, leading to the destruction of SPI aggregates or hindering the formation of more protein aggregates. However, higher screw speeds are beneficial for achieving a more uniform aggregate system of SPI.
1.5.1 Impact of Different Extrusion Temperatures on the Relative Content of SPI Secondary Structure
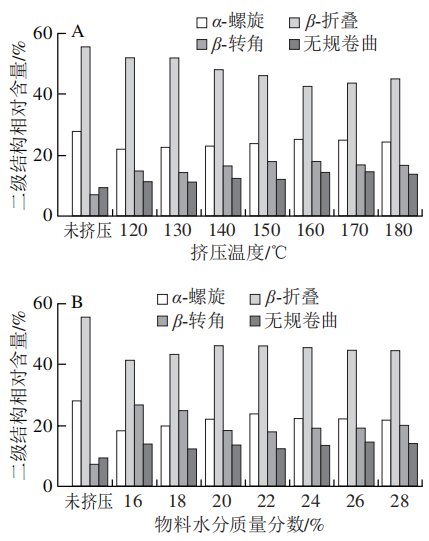
1.5.2 Impact of Different Material Moisture Contents on the Relative Content of SPI Secondary Structure
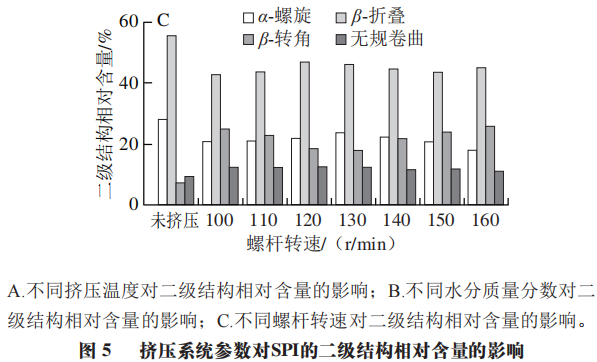
1.5.3 Impact of Different Screw Speeds on the Relative Content of SPI Secondary Structure
After extrusion treatment of soy protein at different screw speeds, the relative content of α-helical and β-folding structures in the secondary structure decreases, while the relative content of β-turn and random coil structures slightly increases. As shown in Figure 5C, with the increase in screw speed, the relative content of α-helical and β-folding structures first increases and then decreases, while the relative content of β-turn shows a trend opposite to that of α-helical and β-folding contents. Based on existing research results, it can be inferred that after extrusion treatment, the degree of denaturation of SPI increases, and the secondary structures of α-helical and β-folding convert into β-turn and random coil structures. When the screw speed ranges from 120 to 130 r/min, the proportions of protein secondary structures are closer to the original state. Being lower or higher than this range will lead to an increase in protein disorder, possibly because excessively low screw speeds result in longer residence times, increasing the degree of protein denaturation, while excessively high screw speeds lead to increased mechanical energy input, causing damage to the natural structure of the protein.
02
Conclusion
This experiment used SPI as the raw material to study the effects of different extrusion temperatures (120-180 °C), screw speeds (100-160 r/min), and material moisture contents (22%-28%) on the conformation of soy protein in cold-pressed soybean meal. The results show that at 150 °C, the protein polymerization degree of the extrudate is higher, and the fiber formation effect is better. Both excessively high and low temperatures are not conducive to protein aggregation and the formation of a stable state; when the material moisture content is between 20% and 22%, the protective effect of water results in the best state of the extrudate, forming a uniform system of SPI aggregates. When the material moisture content is too low or too high, excessive die pressure makes it difficult to form a more complete extrudate; lower screw speeds result in insufficient mechanical energy input and excessive residence time of SPI, preventing complete denaturation and depolymerization of the protein, while higher screw speed conditions promote the destruction of SPI’s original natural structure through high shear forces, forming larger molecular weight protein aggregates. In summary, under appropriate system parameters, extrusion is conducive to obtaining the best quality extrudate; too low results in incomplete protein denaturation, lower polymerization degree, and poor fiber formation effect, while too high will cause excessive protein denaturation, damaging the already formed fiber structure and degrading the quality of the extrudate.
This article “The Impact of Extrusion Parameter Control on the Microstructure of Soy Protein” is sourced from “Food Science”, 2023, Volume 44, Issue 15, pages 103-112, authors: Zhang Haojia, Zhu Xiuqing, Sun Ying. DOI:10.7506/spkx1002-6630-20220721-246. Click below to read the original text for more information about the article.

Intern Editor: Mei Juan; Editor: Zhang Ruimei. Click below to read the original text to view the full text.
Recent Research Hotspots
“Food Science”: Academician Xie Mingyong from Nanchang University et al.: The Influence of Frying Technology on the Formation of Acrylamide and 5-Hydroxymethylfurfural in French Fries
“Food Science”: Associate Professor Chen Pei from Shaanxi Open University et al.: Analysis of Microbial Diversity in Liquor Fermentation
“Food Science”: Professor Sun Dongliang from Beijing University of Petroleum and Chemical Technology et al.: Integrated Mushroom Hot Air Drying Simulation Method and Application
“Food Science”: Researcher Bi Jinfeng from the Chinese Academy of Agricultural Sciences: Research Progress on the Impact of Drying on the Color of Fruit and Vegetable Processing
“Food Science”: Associate Professor Wang Wenxiu from Hebei Agricultural University et al.: Analysis of the Relationship between Protein and Quality Changes during Hot Air Drying of Litopenaeus vannamei
“Food Science”: Senior Engineer Sun Lei from Hebei Provincial Key Laboratory of Food Safety et al.: Determination of 13 Diuretics in Animal-Derived Foods by Ultra-High Performance Liquid Chromatography-Tandem Mass Spectrometry