The peony flower is renowned for its vibrant colors and is known as the “king of flowers”. In China, there has been a long-standing tradition of consuming peony flowers, leading to the creation of various local delicacies, such as peony flower cakes and peony flower tremella soup. In 2013, the Danfeng peony was recognized by the Ministry of Health as a new resource food material, and it has been developed and utilized by numerous researchers.
Research has shown that adding soy protein isolate (SPI) to flour significantly increases the protein and gluten protein content, improving the texture of noodles and noticeably reducing breakage rates. Adding no more than 5% SPI during bread production can enhance the color of the bread crust, increase the bread volume, and improve flavor, with significant nutritional supplementation effects. When applied to cookie production, it can enhance water retention and extend the product’s shelf life.

As a plant material that can be used both as food and medicine, the protein content of peony flower stamen accounts for 23.73% of its dry weight, with a reasonable amino acid composition, where essential amino acids account for 38.26%, making it a high-quality protein resource. Therefore, Professor Luo Lei, Xia Yingli, Yang Haokun, and others from the School of Food and Biological Engineering at Henan University of Science and Technology, as well as the Engineering Technology Research Center for Agricultural Product Drying in Henan Province studied the effects of peony flower stamen protein on dough texture and dynamic rheological properties by comparing different amounts of SPI added, as well as its effects on the disulfide bond content, surface microstructure, and secondary structure of gluten proteins, providing theoretical support for the development and application of peony flower stamen protein in noodle products.
1. Analysis of Dough Texture Properties
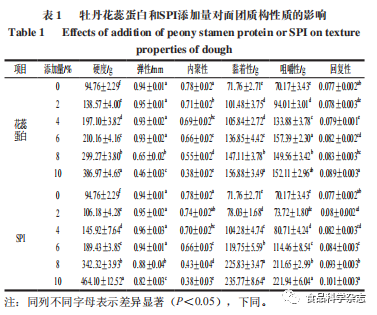
As shown in Table 1, the effects of different amounts of SPI and peony flower stamen protein on dough texture are basically consistent, with the hardness, stickiness, chewiness, and recovery increasing with the amount added. When the amount of peony flower stamen protein added is 6%, the dough hardness, stickiness, and chewiness are 20.73, 17, and 42.93 g higher than the same amount of SPI added, respectively, indicating that peony flower stamen protein is more effective than the same low amount of SPI in enhancing dough properties, while the differences in elasticity, cohesiveness, and recovery compared to SPI are not significant. Elasticity and cohesiveness gradually decrease, especially when the amount exceeds 6%, and the elasticity of the dough with added stamen protein decreases significantly. This may be due to the added protein limiting the formation of disulfide bonds between glutenin, impairing gluten functionality and densifying the internal structure..
2. Analysis of Dynamic Rheological Properties of Dough
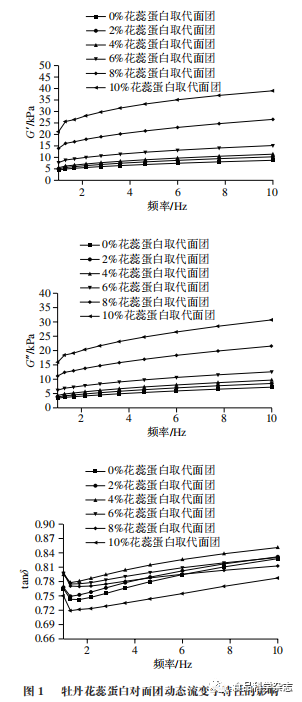
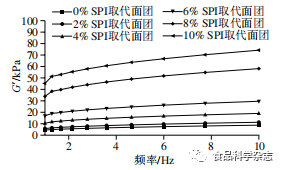
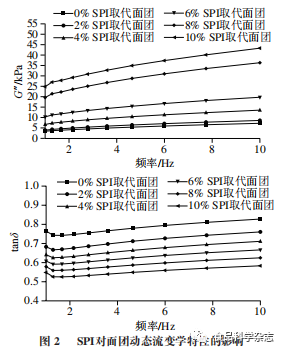
As shown in Figures 1 and 2, the effects of adding peony flower stamen protein and SPI on the rheological properties of dough, G’ and G”, follow a similar pattern, increasing with the amount of protein added. At the same frequency, when the amount of protein added does not exceed 6%, the effects of peony flower stamen protein on dough G’ and G” are comparable to those of soy SPI, which is consistent with the dough texture results. This may be because adding low amounts of protein promotes the interaction of gluten proteins in the dough, forming new cross-linked structures, enhancing the gluten protein network structure, resulting in better viscoelasticity. As shown in the figures, as the amount of peony flower stamen protein increases, tanδ first increases and then decreases, reaching a maximum value when the substitution amount is 4%, indicating that the dough’s viscosity is greater, while the dough with SPI added shows a continuous decrease in tanδ. With the continuous increase in frequency, tanδ in Figures 1 and 2 shows a trend of first decreasing and then increasing. This indicates that at low frequencies, the mixed dough exhibits good viscoelasticity, and as the frequency increases, the viscoelasticity increases rapidly, indicating that at high frequencies, the mixed system structure becomes unstable and is relatively more prone to damage.
3. Analysis of Microstructure of Gluten Protein
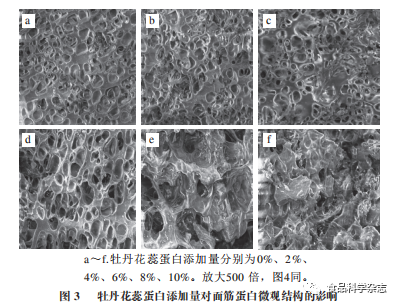
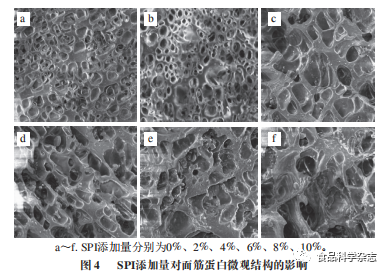
From Figures 3 and 4, it can be seen that the gluten protein without added protein has a surface with intact pores, smoothness, and good continuity, with numerous and uniformly sized pores, but the pores are shallow, resulting in weak gas retention. As the amounts of both proteins increase, the diameter of the gas pores in the gluten protein gradually increases, with many pores deepening. When the amount of protein added is 6%, compared to the microstructure of SPI gluten protein, the gluten protein with flower stamen protein has a greater number of pores, thinner pore walls, and better continuity. This indicates that the ability of flower stamen protein to interact with gluten protein is greater than that of SPI, allowing the alcohol-soluble protein to bind with glutenin through non-covalent bonds, promoting the formation of the gluten network structure. As the amount of protein continues to increase, it can be observed that the orderliness of both gluten proteins significantly weakens, and compared to the control group, the surface smoothness and uniformity deteriorate, with pore sizes increasing, fractures occurring, and pore walls thickening, causing the gluten protein structure to become loose. Especially when the amount of peony flower stamen protein reaches 10%, the internal structure of the gluten protein is completely destroyed, likely due to the strong water absorption of the added protein, where higher amounts of protein compete for water with gluten protein, which is unfavorable for the formation of the spatial network structure of gluten protein..
4. Analysis of Secondary Structure of Gluten Protein
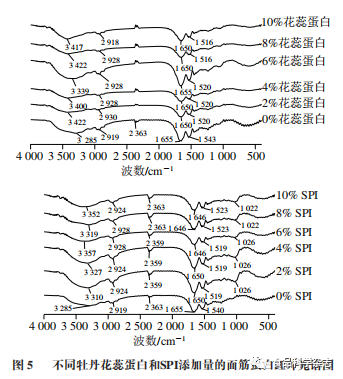
As shown in Figure 5, at 3100-3500 cm-1, there is an absorption band. Both peony flower stamen protein and SPI showed a red shift compared to the control group, which is related to the O—H stretching vibration absorption of carbohydrates and the connection of intermolecular hydrogen bonds. From Figure 5, it can be seen that absorption peaks appear at 2900-3000, 1600-1700 cm-1, and 1510-1500 cm-1, and the positions have shifted, indicating that added proteins affect gluten protein. The amide I band (mainly C=O stretching vibration, 1600-1700 cm-1) contains information about α-helix, β-fold, β-turn, and random coil structures, and is therefore commonly used to analyze the secondary structure of proteins.
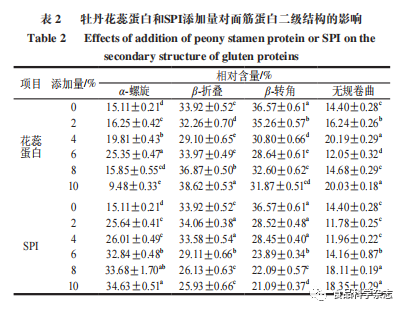
From Table 2, it can be seen that when the amount of flower stamen protein added is 6%, the relative content of α-helix reaches its maximum value, increasing by 10.24% compared to the blank group, while the relative content of α-helix in SPI continuously increases, with a 19.52% increase compared to the blank group when the amount added is 10%. The proportions of β-fold and β-turn structures first decrease and then increase with the addition of peony flower stamen protein, while showing a downward trend with increasing SPI, which may be due to the strong water absorption of the proteins, leading to a continuous reduction of free water in the system, thus damaging the hydrogen bonds in gluten protein. The random coil structure can cause protein conformation instability, and the addition of stamen protein shows irregularity, while the content of SPI in gluten protein continues to increase, indicating that excessive protein addition can compromise the stability of the gluten protein network structure. As the amount of protein added increases, the secondary structure of gluten protein with peony flower stamen protein mainly shifts from β-turn to β-fold, while the secondary structure of gluten protein with SPI shifts from β-fold to α-helix. When the amounts of peony flower stamen protein and SPI are both at 6%, the proportions of α-helix + β-fold reach their maximum values of 59.32% and 61.95%, respectively. Both α-helix and β-fold are relatively stable structures that help improve the hardness and elasticity of noodle products..
5. Analysis of Thiol and Disulfide Bonds in Gluten Protein
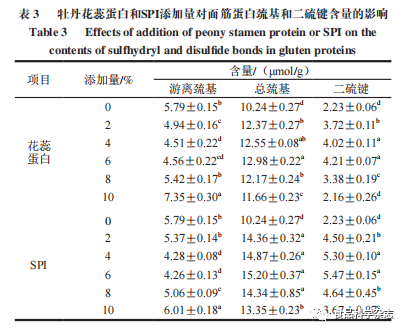
As shown in Table 3, the effects of the two proteins on the disulfide bonds of gluten protein are basically consistent, but SPI is slightly better than peony flower stamen protein. As the amount added increases, the free thiol content first decreases and then increases, while the total thiol and disulfide bond content show a trend of first increasing and then decreasing, which is due to the mutual transformation between disulfide bonds and thiols through redox reactions. When the amount added is 6%, the disulfide bond content of both peony flower stamen protein and SPI increases by 1.98 μmol/g and 3.24 μmol/g, respectively, compared to the control group, indicating that adding an appropriate amount of protein can promote the formation of disulfide bonds in gluten protein, maintaining the stability of the spatial structure.
Peony flower stamen protein can significantly improve the hardness, stickiness, and chewiness of dough, and is more effective than SPI when added below 6%, while the differences in elasticity, cohesiveness, and recovery compared to SPI are not significant. Rheological studies indicate that the addition of protein increases dough G’ and G”, with the maximum tanδ of gluten protein occurring when the amount of flower stamen protein added is 4%, indicating greater dough viscosity. Both low amounts of flower stamen protein and SPI mixed dough exhibit good viscoelasticity.
Scanning electron microscopy shows that the addition of flower stamen protein can improve the gluten network structure, with the best continuity of the network structure at 6% addition, having numerous and deep pores, showing slightly better effects than SPI. The addition of flower stamen protein and SPI can promote the conversion of the unstable β-turn into the stable α-helix and β-fold structures, with the maximum proportions of α-helix + β-fold reaching 59.32% and 61.95% at 6% addition of flower stamen protein. When the addition of peony flower stamen protein is at 6%, the disulfide bond content of gluten protein reaches its highest, increasing by 89% compared to the blank group, with SPI having a slightly better effect on disulfide bond content than flower stamen protein.
This article “Effects of Peony Flower Stamen Protein on Dough and Gluten Properties” is sourced from “Food Science”, 2023, Volume 44, Issue 4, Pages 42-47, authors: Luo Lei, Xia Yingli, Yang Haokun, Zhao Yifan, Li Hanshu, Ma Xiao. DOI:10.7506/spkx1002-6630-20220314-155. Click below to read the original article for more information.
“Food Science”: Professor Chen Ji-wang from Wuhan University of Light Industry and others: The effects of wheat starch and gluten interaction on the oil distribution of fried batter-coated catfish fillets.
“Food Science”: Professor Zhang Na from Harbin University of Commerce and others: Preparation of tamarind seed globulin-EGCG covalent complex and its application in emulsified sausages.
“Food Science”: Dr. Chen Cheng from Gansu Agricultural University and others: Active oxygen activates hypoxia-inducible factor-1α to accelerate energy metabolism in the early stage of post-mortem beef maturation.
“Food Science”: Dr. Chen Cheng from Gansu Agricultural University and others: Active oxygen activates hypoxia-inducible factor-1α to accelerate energy metabolism in the early stage of post-mortem beef maturation.
“Food Science” Plant-based Meat Column: Professor Zhu Xiuqing from Harbin University of Commerce and others: Research progress on the flavor influence of soybean protein meat analogs.
“Food Science” Plant-based Meat Column: Academician Chen Jian from Jiangnan University and others: The effect of glutamine transaminase on the structure and digestion characteristics of high-moisture pea protein extrudates.
“Food Science” Plant-based Meat Column: Researcher Wang Qiang from the Chinese Academy of Agricultural Sciences and others: Research progress on enzyme modification technology and its application in plant-based meat products.
“Food Science”: Associate Professor Cheng Nan from China Agricultural University and others: Research progress of paper-based micro-laboratories in food testing.
“Food Science”: Associate Professor Liu Jun from Jiangxi Normal University and others: Mechanism of glycosylation combined with phosphorylation in reducing the allergenicity of catfish albumin.
“Food Science”: Professor Zou Xiaobo from Jiangsu University and others: High stability freshness visualization indicator film based on acylated anthocyanins and its application.
“Food Science”: Associate Professor Chen Bingzhi from Fujian Agriculture and Forestry University and others: The effect of ultrasonic treatment on the storage quality of deer antler mushrooms.
“Food Science”: Professor Sun Tong from Bohai University and others: The effect of sandwich-type konjac glucomannan/sodium alginate/konjac glucomannan composite preservation film on protein oxidation of salmon fillets.
“Food Science”: Professor Liu Haijie from China Agricultural University and others: CaCl2 electrolysis treatment improves the quality of mung bean sprouts and its mechanism.
“Food Science”: Professor Sun Tong from Bohai University and others: The effect of sandwich-type konjac glucomannan/sodium alginate/konjac glucomannan composite preservation film on protein oxidation of salmon fillets.
“Food Science”: Professor Liu Haijie from China Agricultural University and others: CaCl2 electrolysis treatment improves the quality of mung bean sprouts and its mechanism.
Edited by: Yuan Yi; Chief Editor: Zhang Ruimei. Click below to read the full text.
Images sourced from the original article and Shutterstock.
 In order to build a diversified food supply system while considering ecological and environmental protection, and to promote the sustainability of food production through biodiversity conservation, the Beijing Food Science Research Institute and the China Food Magazine will jointly hold the “International Symposium on Ecological Protection and Sustainable Development of Food” with Northern Minzu University, Anhui Xiyuan University, Suzhou University, and Chuzhou University from May 13-14, 2023 in Yinchuan, Ningxia, China. This symposium will focus on the current status of food resource development, including the exploration of new resource foods, plant and animal proteins, microbial alternatives, edible fungi, important innovations, and existing problems, exploring future food development directions, showcasing the latest research achievements in ecological protection and sustainable food development in China, and building a platform for collaboration between research institutions and enterprises to jointly promote the rapid advancement of China’s food industry into a new phase.
In order to build a diversified food supply system while considering ecological and environmental protection, and to promote the sustainability of food production through biodiversity conservation, the Beijing Food Science Research Institute and the China Food Magazine will jointly hold the “International Symposium on Ecological Protection and Sustainable Development of Food” with Northern Minzu University, Anhui Xiyuan University, Suzhou University, and Chuzhou University from May 13-14, 2023 in Yinchuan, Ningxia, China. This symposium will focus on the current status of food resource development, including the exploration of new resource foods, plant and animal proteins, microbial alternatives, edible fungi, important innovations, and existing problems, exploring future food development directions, showcasing the latest research achievements in ecological protection and sustainable food development in China, and building a platform for collaboration between research institutions and enterprises to jointly promote the rapid advancement of China’s food industry into a new phase.
 Long press or scan the QR code to register
Long press or scan the QR code to register



Food Science of Animal Products(ISSN: 2958-4124, e-ISSN : 2958-3780)is an international peer-reviewed, open-access journal sponsored by the Beijing Food Science Research Institute and the China Meat Products Comprehensive Research Center, operated by the editorial team of the magazine “Food Science”, which belongs to the field of food science and technology, aiming to report the latest research findings in the field of animal-derived foods, including meat, aquatic products, dairy, eggs, animal offal, edible insects, etc. The research content includes the quality of food raw materials, processing characteristics, the relationship between nutritional components, active substances, and human health, product flavor and sensory characteristics, control of harmful substances in processing or cooking, product preservation, storage and packaging, microbiology and fermentation, illegal drug residues and food safety testing, authenticity verification, cell-cultured meat, regulatory standards, etc.
Submission website:
https://www.sciopen.com/journal/2958-4124