
01
Introduction
Research has confirmed that GABAergic neurons regulate synaptic excitability by activating presynaptic GABAB receptors (GABABRs). However, recent studies have shown that astrocytes also express GABABRs and can respond to the activity of GABAergic neurons. Nevertheless, it has been challenging for researchers to distinguish or overlook whether astrocytic GABABRs are involved in the mechanisms by which GABAergic neurons regulate synaptic excitability. In this study, we employed techniques such as whole-cell patch-clamp recording, optogenetics, calcium imaging, and pharmacology to confirm that SOM-INs participate in excitatory synaptic regulation through astrocytic GABABRs and reveal the underlying mechanisms.
02
Original Information

03
Main Content
GABAergic interneurons are crucial for coordinating excitatory patterns and synchronizing neuronal network activity in the brain. Among them, somatostatin (SOM)-expressing interneurons are a type of interneuron that targets the distal dendritic regions of pyramidal neurons, and the GABA they release acts on presynaptic GABAB receptors (GABABRs) to inhibit synaptic transmission.
Astrocytes sense changes in their environment through various membrane receptors they express. Reports have confirmed that the activity of GABAergic interneurons can induce astrocytes to release ATP/Adenosine, thereby activating presynaptic Adenosine A1 receptors (A1R) and inhibiting excitatory synaptic transmission. However, it remains unclear which type of interneuron is involved in these processes. Additionally, recent findings suggest that the activation of SOM-INs can enhance Ca2+ signaling in astrocytes. Therefore, we hypothesize that the purinergic signaling induced by the activation of astrocytic GABABRs is involved in the regulation of excitatory synaptic transmission mediated by SOM-INs.
To test our hypothesis, we used a viral method for stereotaxic injection to express the light-sensitive protein ChR2 in SOM-INs in the hippocampus. We found that blue light stimulation of SOM-INs led to a reduction in CA3-CA1 excitatory synaptic transmission, and this phenomenon could be blocked by a GABABR antagonist, indicating the involvement of GABABRs in this process (Figure 1).
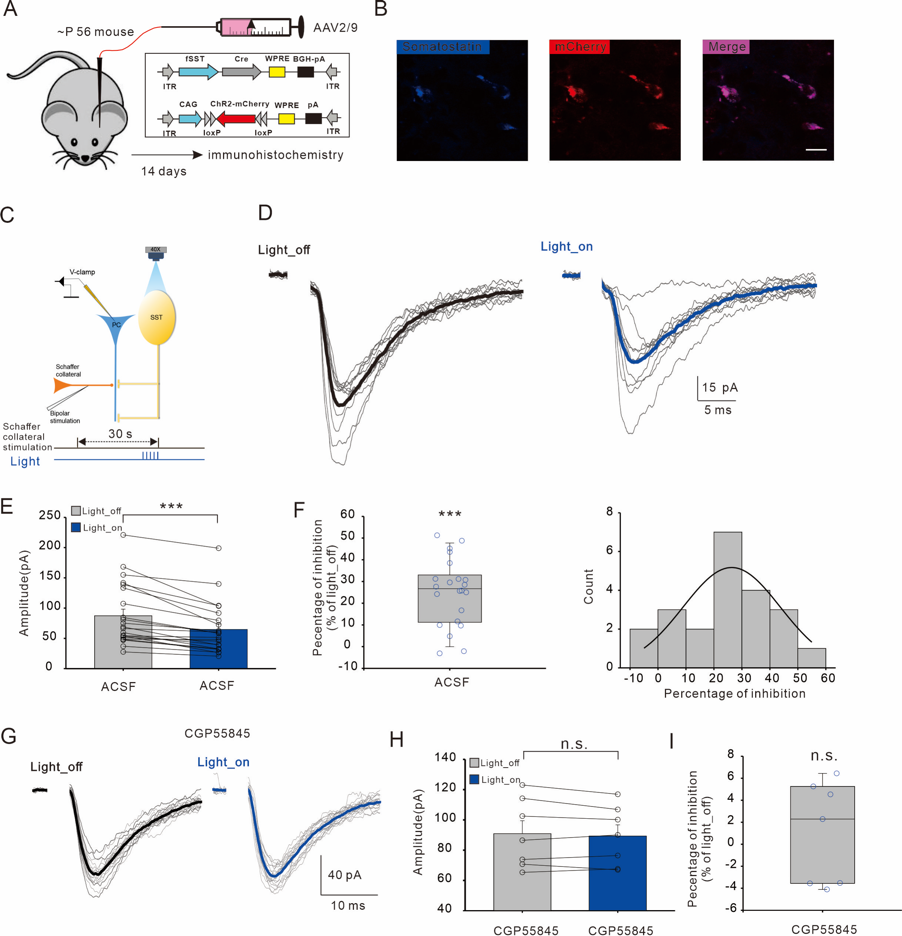
FIG1
GABAB receptors are involved in the inhibition of excitatory synaptic transmission caused by optogenetic activation of SOM-INs.
Next, we combined optogenetics and Ca2+ imaging to discover that blue light activation of SOM-INs enhances Ca2+ signaling in astrocytes. Subsequent pharmacological experiments revealed that astrocytic GABABRs mediated this process (Figure 2).
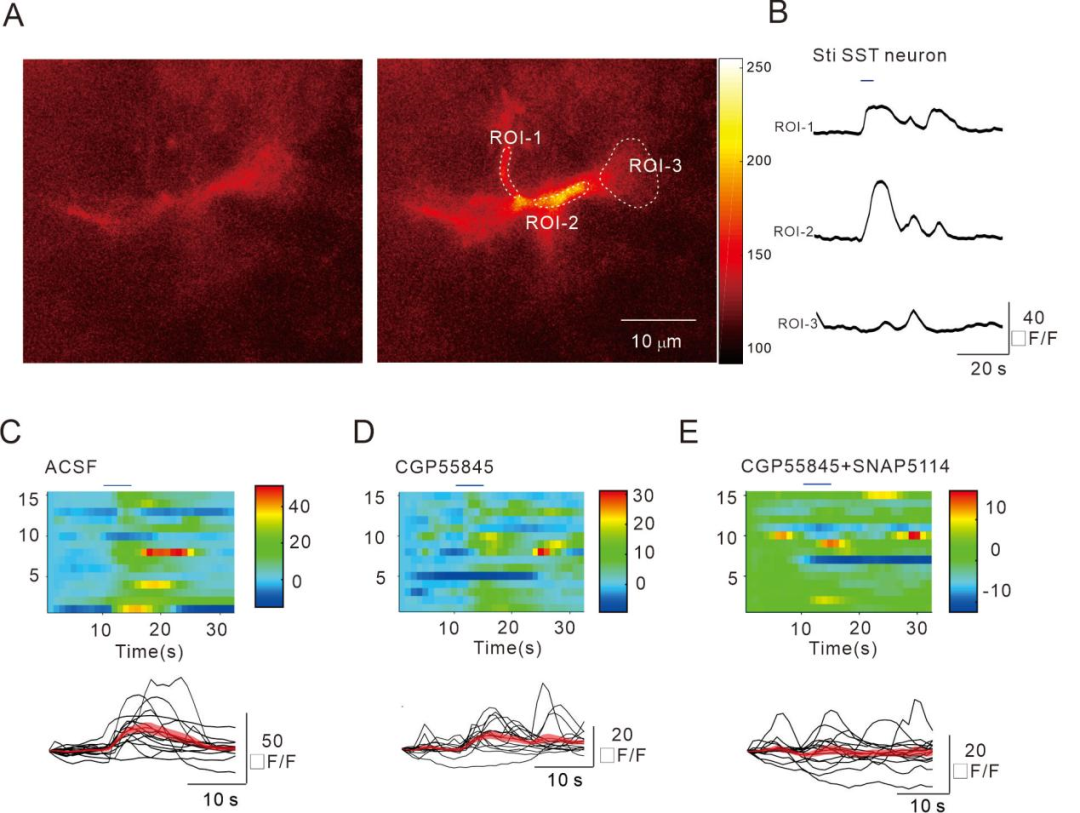
FIG2
SOM-INs enhance intracellular Ca2+ signaling in astrocytes through the activation of GABABRs.
Subsequently, we confirmed the involvement of astrocytes in the reduction of synaptic transmission induced by SOM-INs activation through experiments that clamp astrocytic Ca2+ concentrations (Figure 3).
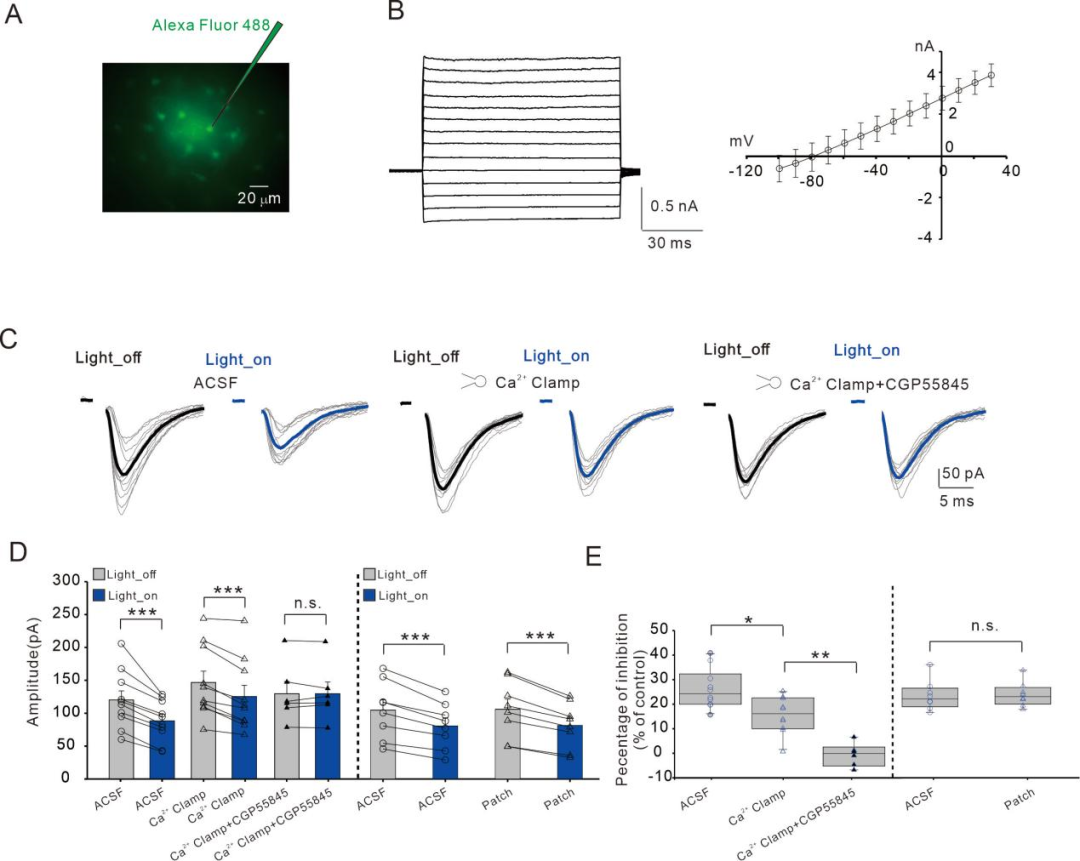
FIG3
Astrocytic Ca2+ signaling is involved in the inhibition of excitatory synapses induced by SOM-INs activation.
Finally, through pharmacological and electrophysiological techniques, we elucidated that the neurotransmitters released by astrocytes (ATP/Adenosine) participate in this process by acting on presynaptic A1Rs (Figure 4).
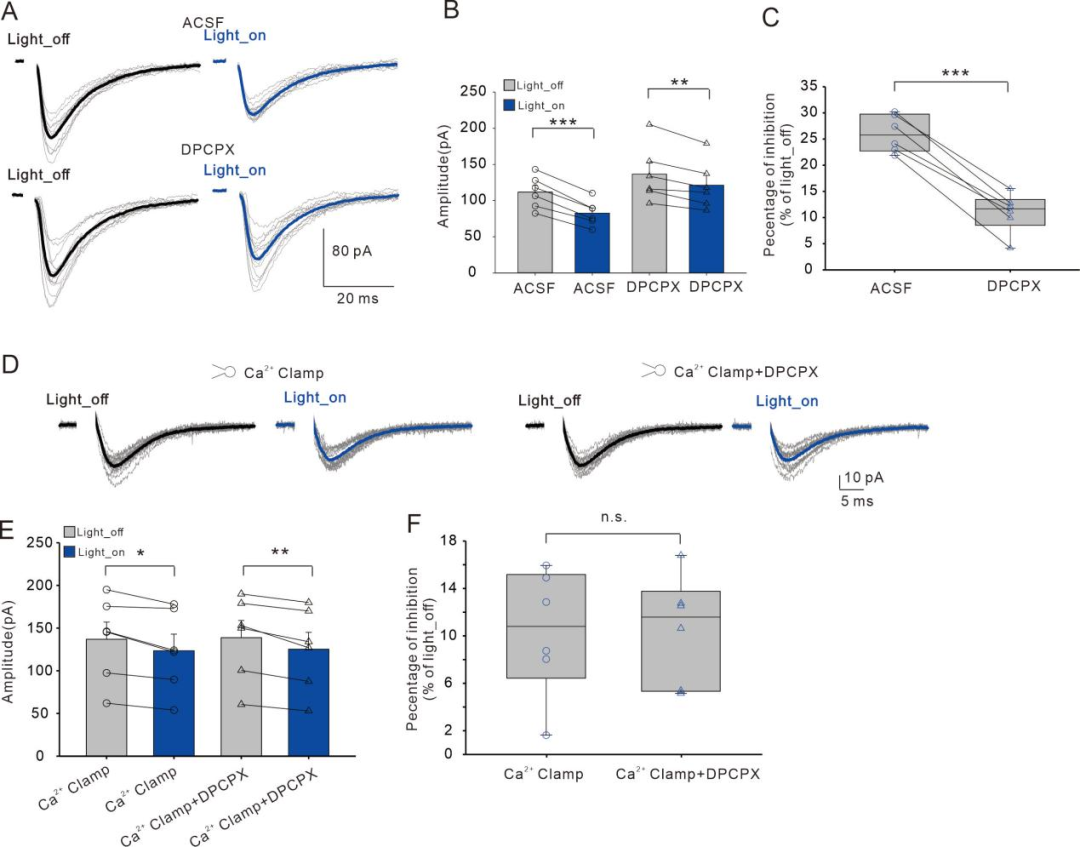
FIG4
SOM-INs inhibit synaptic transmission through astrocyte-mediated ATP/Adenosine signaling acting on presynaptic A1Rs.
04
Results
The results indicate that the GABA released by SOM-INs can act on astrocytic GABABRs, leading to an increase in intracellular Ca2+ signaling in astrocytes. Furthermore, we found that the increase in Ca2+ signaling induced by the activation of astrocytic GABABRs can induce astrocytes to release ATP/Adenosine, which then acts on presynaptic A1Rs to inhibit synaptic transmission in the hippocampal CA3-CA1 pathway. This study provides a novel perspective on the mechanisms by which SOM-INs regulate synaptic transmission.
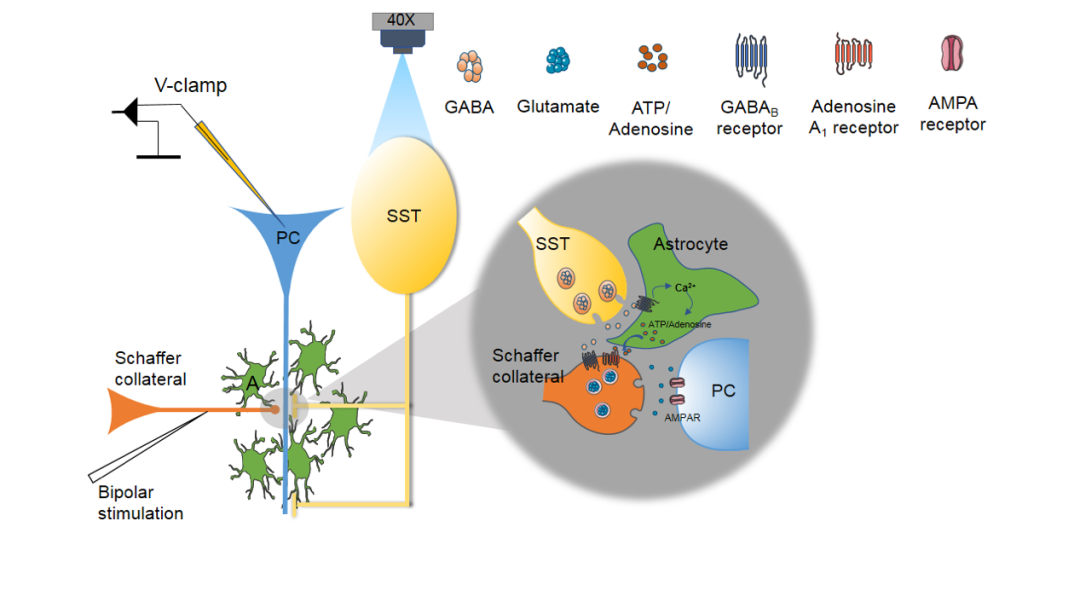
FIG5
Diagram of the signaling pathway by which hippocampal SOM-INs regulate excitatory synaptic transmission.
Professor Zeng Linghui and Teacher Shen Weida from the Medical School of Zhejiang University City College are the corresponding authors of this article. Graduate students Li Zijing, Tang Yejiao, and undergraduate student Han Pufan from Zhejiang University City College are the co-first authors of this article. This research was funded by the Zhejiang Provincial Key Research and Development Program (20220C03034) and the National College Student Innovation and Entrepreneurship Training Program (202213021026).
Article edited by:
Shen Weida | Zhejiang University City College, PhD
Research direction: Neuron-glia interactions in health and disease
Follow us on the “Wiley NeuroPsycho” WeChat official account or
Contact us: [email protected]
Article reproduced from: Wiley NeuroPsycho