Source: Professor Zhang Bo
The activation mechanisms of the HER-2 gene include amplification, mutation, insertion, and protein overexpression. Although HER-2 is considered a driver gene in lung cancer, no targeted therapeutic drugs have been approved. Recently, Lancet Oncology published efficacy and safety data for the ADC drug DS-8201 targeting HER2 in patients with advanced NSCLC who overexpress HER-2 protein.
Research Background
The DESTINY-Lung01 study is a multicenter, open-label, phase II clinical trial aimed at exploring the efficacy and safety of the HER-2 antibody-drug conjugate DS-8201 in patients with metastatic non-small cell lung cancer (NSCLC) who overexpress or have mutations in HER-2. Efficacy and safety data for the HER-2 mutation cohort have been reported, and this article aims to explore the efficacy and safety of different doses of DS-8201 in patients with HER-2 protein overexpression in NSCLC.
Research Methods
This study included patients aged 18 years or older with histologically confirmed unresectable or metastatic NSCLC, who progressed after standard treatment and currently had no standard treatment options available. HER-2 protein overexpression was assessed using immunohistochemistry, with scores of 2+ or 3+, and without HER-2 gene mutations, with a PS score of 0-1. Eligible patients were sequentially assigned to receive either 6.4 mg/kg or 5.4 mg/kg of the drug, administered once every three weeks. The primary endpoint of the study was the overall response rate (ORR).
Research Results
Baseline Characteristics:
From August 2018 to January 2020, 49 patients were included in the high-dose group, with a median age of 63 years, and 61% were male; from July to December 2020, 41 patients were included in the low-dose group, with a median age of 62 years, and 54% were male. In the high-dose and low-dose groups, the proportions of patients with a score of 3+ were 20% and 41%, respectively, and the proportions of patients with brain metastases were 35% and 29%, respectively. 73% and 80% of patients had previously received immune checkpoint inhibitor treatment, and 29% and 17% had received targeted therapy for HER-2 or EGFR, respectively.
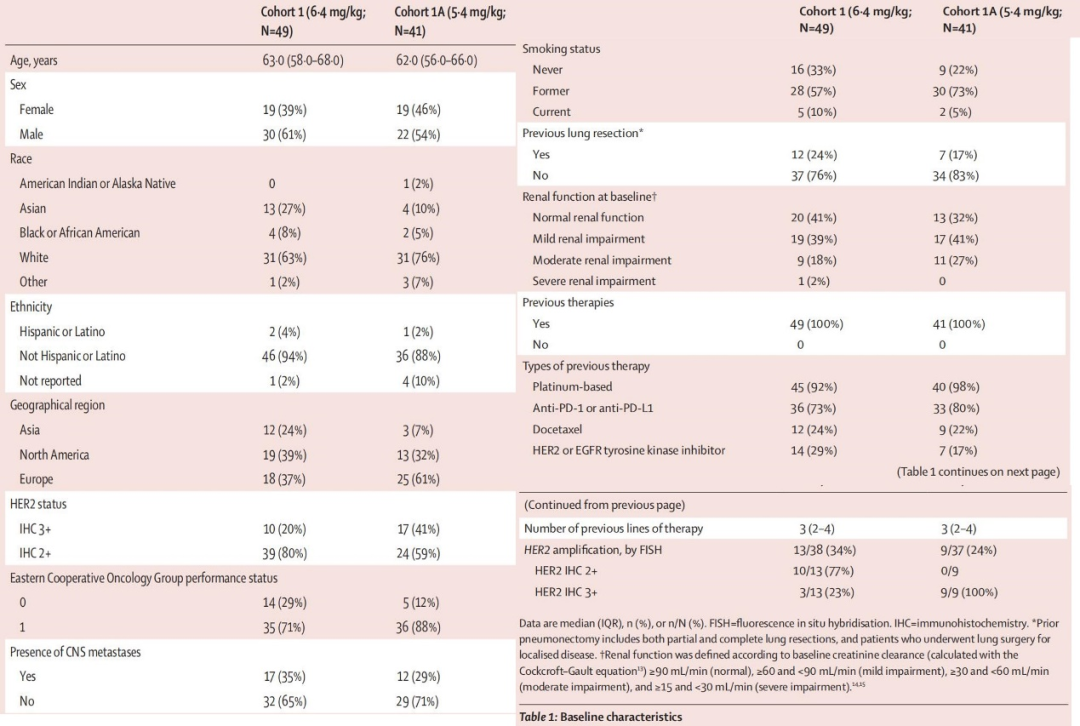 Baseline Characteristics of Patients
Baseline Characteristics of Patients
Efficacy:
As of the data cutoff, the treatment durations for the two groups were 4.1 months and 5.5 months, with follow-up times of 12.0 months and 10.6 months, respectively. The ORR for the high-dose and low-dose groups was 26.5% and 34.1%, both classified as partial responses (PR), with median response durations of 5.8 months and 6.2 months, respectively. The disease control rates (DCR) for the two groups were 69.4% and 78.0%.
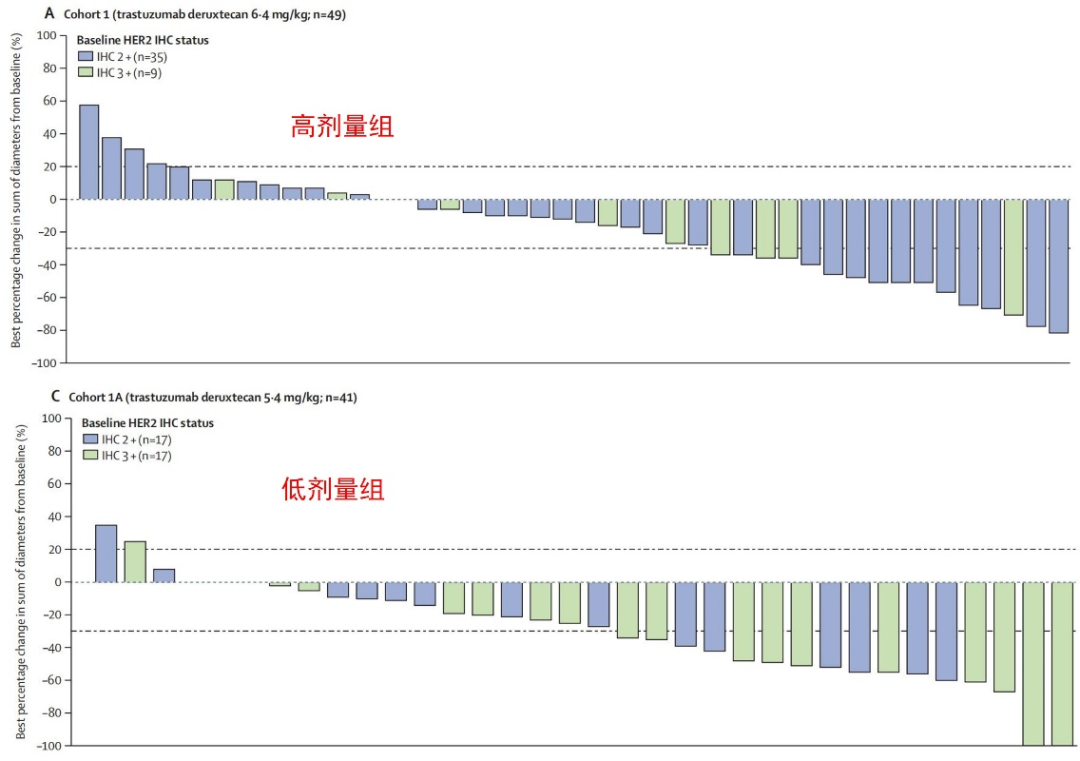 ORR for Both Groups
ORR for Both Groups
The median progression-free survival (PFS) was 5.7 months and 6.7 months, and the median overall survival (OS) was 12.4 months and 11.2 months, respectively.
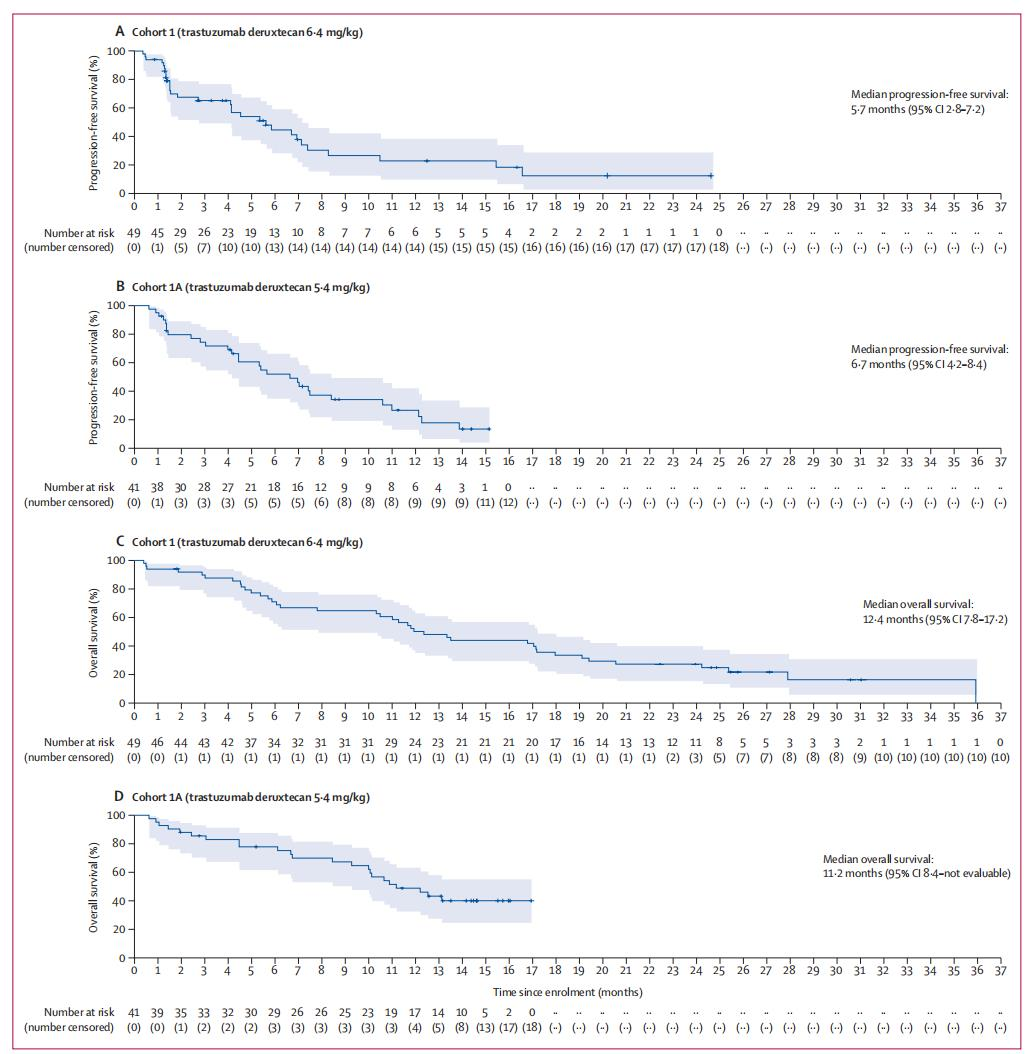 PFS and OS Data
PFS and OS Data
Safety:
The most common grade 3 or higher adverse events and their incidence rates were neutropenia (24% vs. 0%), pneumonia (12% vs. 5%), and fatigue (12% vs. 7%). The incidence rates of drug-related grade 3 or higher adverse events were 53% and 22%. The incidence rates of drug-related serious adverse events were 20% and 7%, respectively. The incidence rates of drug-related interstitial lung disease or pulmonary disease were 20% (grade 1: 4%; grade 2: 10%; grade 5: 6%) and 5% (grade 2: 2%; grade 5: 2%), respectively. In the low-dose group, there was one additional patient assessed with grade 4 pneumonia, which progressed to grade 5 pneumonia after the data cutoff.
Research Conclusion
Considering that existing treatments have low antitumor activity after standard treatment in this patient population, DS-8201 may have the potential to meet the treatment needs of these patients. This study supports further exploration.
References
Smit EF, Felip E, Uprety D, et al. Trastuzumab deruxtecan in patients with metastatic non-small-cell lung cancer (DESTINY-Lung01): primary results of the HER2-overexpressing cohorts from a single-arm, phase 2 trial. Lancet Oncol. 2024;25(4):439-454. doi:10.1016/S1470-2045(24)00064-0
Editor: Nydia Layout Editor: Nydia
Copyright Statement: This article is intended for reference by medical professionals only and may not be published or distributed without the author’s permission. Individuals are welcome to share it, but any other media or websites wishing to reproduce or quote content owned by this website must obtain authorization and prominently indicate “Reprinted from: Liangyi Hui – Oncology Doctor APP”.