
Introduction
Antibody-drug conjugates (ADCs) typically consist of: highly specific and high-affinity antibodies, stable linkers, and potent small-molecule cytotoxic drugs1. The key to the development of ADCs lies in the selection of targets, antibodies, cytotoxic payloads, linkers, and the conjugation method. Among these, the conjugation method of ADCs directly determines the drug-to-antibody ratio (DAR), the distribution of conjugation sites, and the stability of the conjugation, among other properties. This is also the most challenging barrier in the technological development of ADCs. This article will focus on the conjugation methods of ADCs.
Previous Articles:
ADC Series – Target Section
ADC Series – Antibody Section
ADC Series – Linker Section
ADC Series – Payload Section

Figure 1 Schematic of ADC Structure
Classification of ADC Conjugation Methods
The first type is non-specific conjugation technology (random conjugation technology) : lysine-based conjugation technology and cysteine-based conjugation technology;The second type is specific conjugation technology: introducing reactive cysteine conjugation (Thio-mab) technology, interchain disulfide bond modification, unnatural amino acid conjugation technology, enzyme modification conjugation, etc..2-3
Non-Specific Conjugation Technology

Figure 2 A Lysine Residue Conjugation and Its DAR Distribution; B Cysteine Residue Conjugation and Its DAR Distribution
Specific Conjugation Technology

Figure 3 THIOMAB Technology Process

Figure 4 Re-bridging of Interchain Disulfide Bonds

Figure 5 Process of Unnatural Amino Acid Conjugation
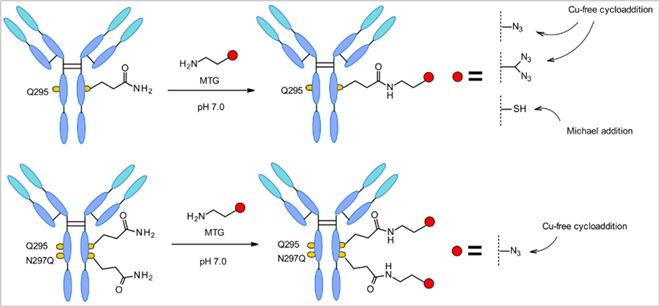
Figure 6 Microbial Transglutaminase (MTGase) Method
Conclusion

Table 1 Characteristics of Common Conjugation Methods for ADCs
References
1. Chinese Anti-Cancer Association Clinical Research Professional Committee of Tumor Drugs, National Expert Committee for Clinical Application Monitoring of Anti-Cancer Drugs, National Tumor Quality Control Center Breast Cancer Expert Committee, et al. Expert Consensus on the Clinical Application of Antibody-Drug Conjugates for Malignant Tumors in China (2023 Edition) [J]. Chinese Journal of Oncology, 2023, 45(9):741-762.
2. Li M, Zhao X, Yu C, et al. Antibody-Drug Conjugate Overview: a State-of-the-art Manufacturing Process and Control Strategy. Pharm Res. 2024 Mar;41(3):419-440.
3. Tsuchikama K, An Z. Antibody-drug conjugates: recent advances in conjugation and linker chemistries. Protein Cell. 2018 Jan;9(1):33-46.
4. Metrangolo V, Engelholm LH. Antibody-Drug Conjugates: The Dynamic Evolution from Conventional to Next-Generation Constructs. Cancers (Basel). 2024 Jan 20;16(2):447.
5. Fu Z, Li S, Han S, et al. Antibody drug conjugate: the “biological missile” for targeted cancer therapy. Signal Transduct Target Ther. 2022 Mar 22;7(1):93.
6. Kostova V, Désos P, Starck JB, et al. The Chemistry Behind ADCs. Pharmaceuticals (Basel). 2021 May 7;14(5):442.
* This article is for the purpose of providing scientific information to medical professionals and does not represent the views of this platform.


