
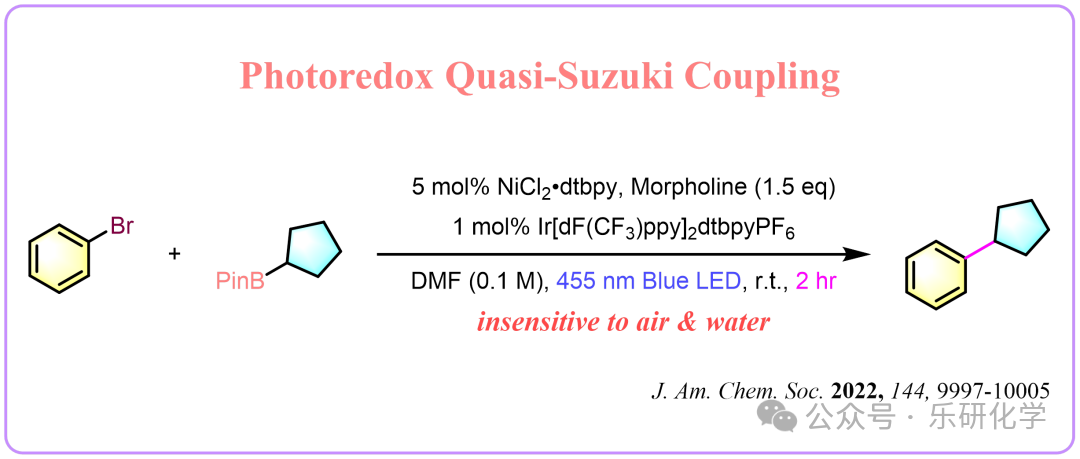
 From J. Am. Chem. Soc.
From J. Am. Chem. Soc.
Hi, everyone! As chemists, what does your ideal coupling reaction look like? I believe it should be like this: first, the reaction should have a simple system; second, the reagents should have no absorption and not interfere with monitoring; then, the reaction should not be sensitive to water or oxygen; finally, the reaction conditions should be mild and safe. Is there such a reaction? Of course, there is. Let’s take a look at the following:
“Perfect Flawless”—— Quasi-Suzuki Coupling Mechanism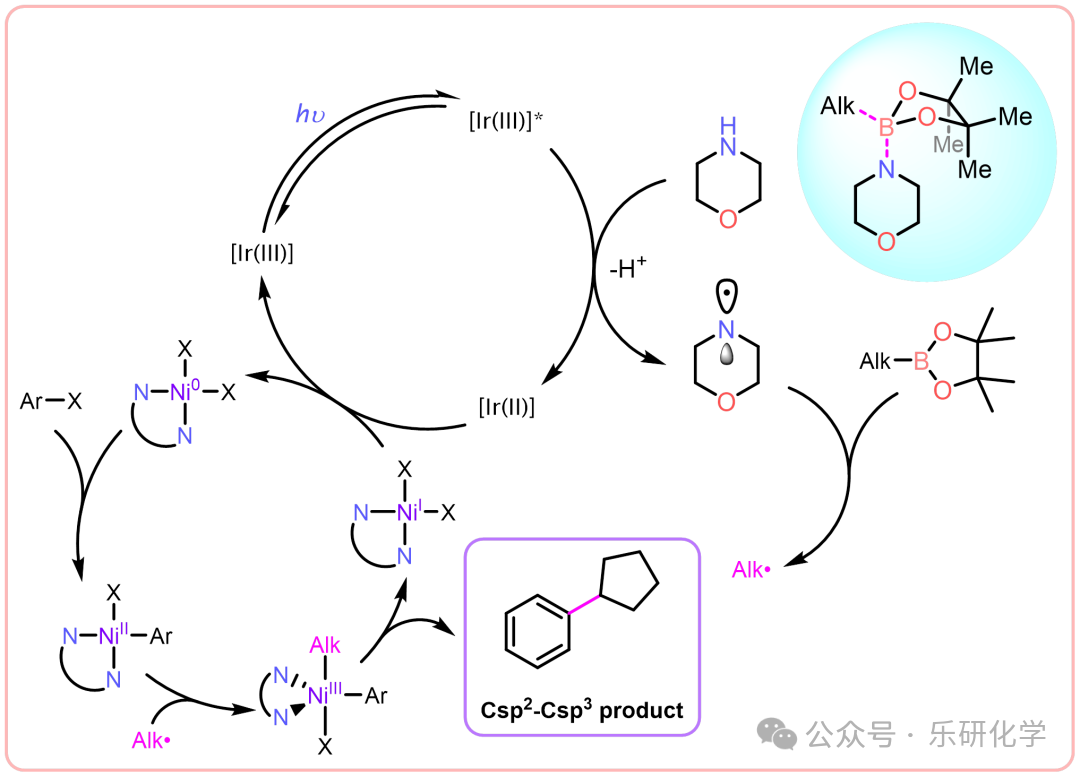 Substrate Expansion
Substrate Expansion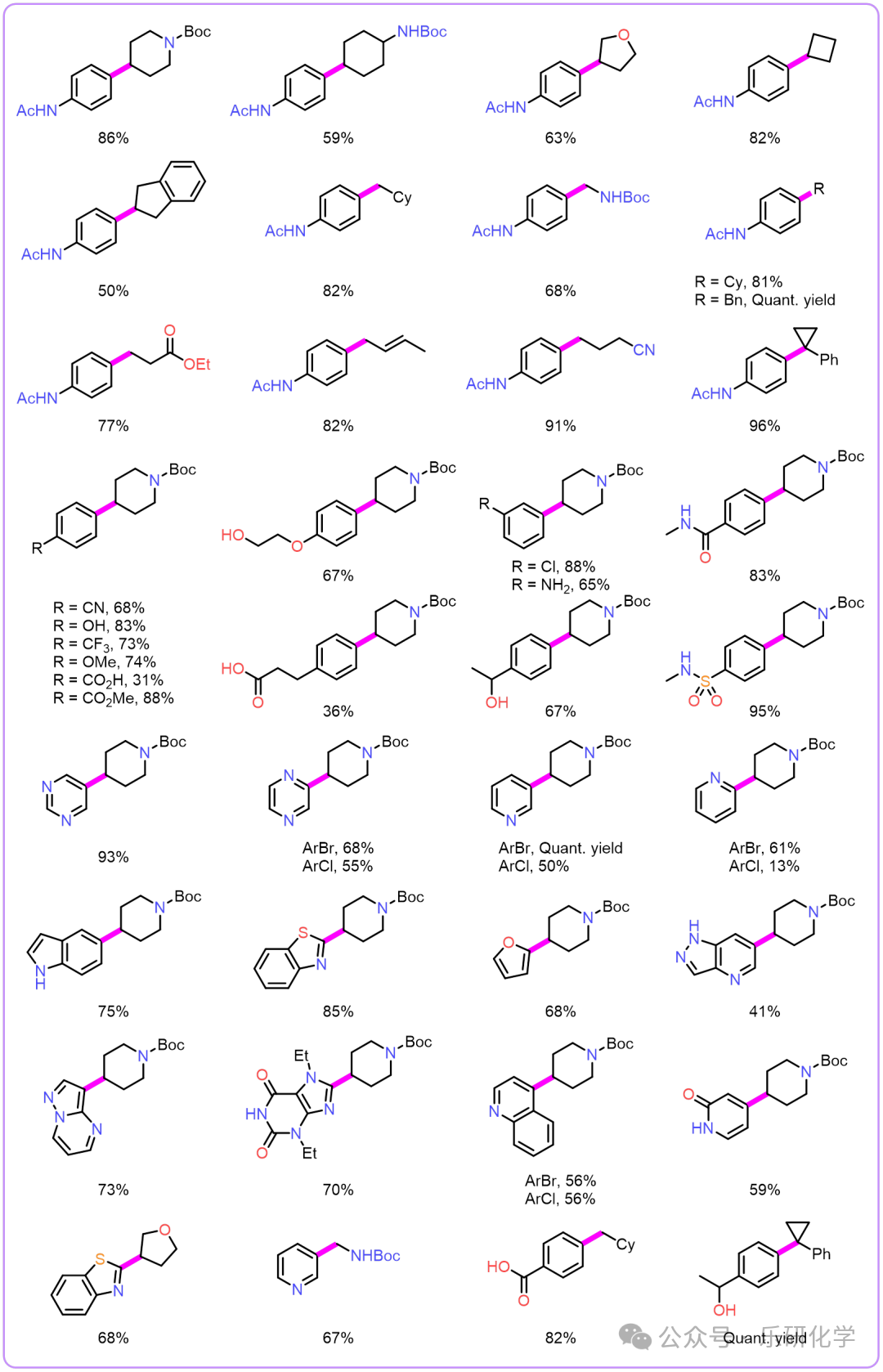 Highlights[1] The most perfect Csp²- Csp³ photocatalytic coupling reaction! Simple system, mild conditions, inexpensive reagents, tolerant to water and oxygen, and the reaction system is homogeneous, allowing for fluid photochemistry to be directly released.;[2] This method has good functional group tolerance, being compatible with alcohol hydroxyl, phenolic hydroxyl, carboxyl, aromatic amines, sulfonamides, cyano groups, trifluoromethyl, ester groups, etc.;[3] This method has a wide substrate range, including but not limited to benzene, pyridine, indole, quinoline, etc.;[4] The range of aryl halide substrates is extensive, including benzene rings, pyridine, pyrimidine, pyrazine, quinoline, furan, benzothiazole, purine, etc.;
Highlights[1] The most perfect Csp²- Csp³ photocatalytic coupling reaction! Simple system, mild conditions, inexpensive reagents, tolerant to water and oxygen, and the reaction system is homogeneous, allowing for fluid photochemistry to be directly released.;[2] This method has good functional group tolerance, being compatible with alcohol hydroxyl, phenolic hydroxyl, carboxyl, aromatic amines, sulfonamides, cyano groups, trifluoromethyl, ester groups, etc.;[3] This method has a wide substrate range, including but not limited to benzene, pyridine, indole, quinoline, etc.;[4] The range of aryl halide substrates is extensive, including benzene rings, pyridine, pyrimidine, pyrazine, quinoline, furan, benzothiazole, purine, etc.;
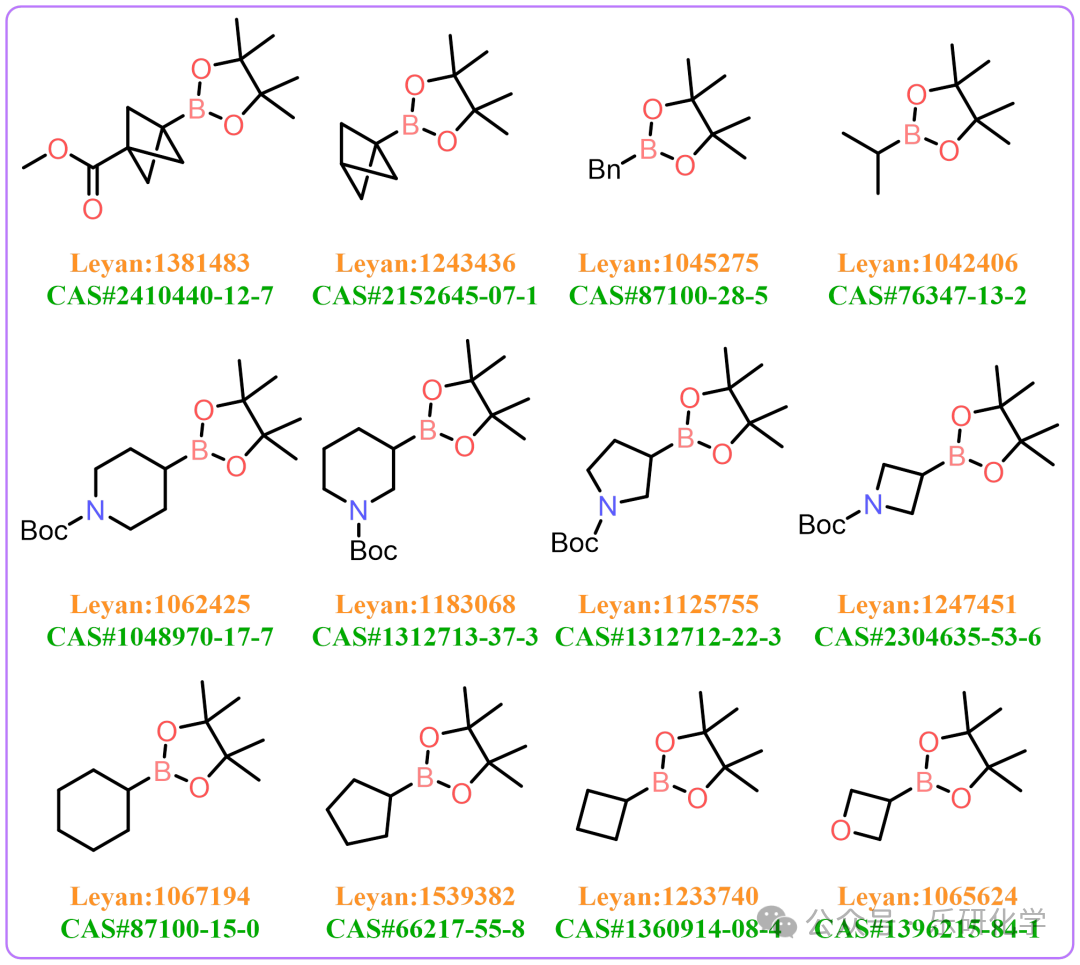
[5] A large number of Alk-BPin have been commercialized, and there are many types of alkyl boronic acid ester building blocks. Some common alkyl boronic ester information is shown in the figure..
 ReferencesART─An Amino Radical Transfer Strategy for C(sp²)–C(sp³) Coupling Reactions, Enabled by Dual Photo/Nickel Catalysis.Elisabeth Speckmeier, Thomas C. MaierJ. Am. Chem. Soc. 2022, 144, 22, 9997–10005.DOI : 10.1021/jacs.2c03220
ReferencesART─An Amino Radical Transfer Strategy for C(sp²)–C(sp³) Coupling Reactions, Enabled by Dual Photo/Nickel Catalysis.Elisabeth Speckmeier, Thomas C. MaierJ. Am. Chem. Soc. 2022, 144, 22, 9997–10005.DOI : 10.1021/jacs.2c03220