|
With the rapid development of 3D printing technology, photopolymers have become important materials for manufacturing complex structures. However, traditional photopolymers (such as acrylates) form irreversible C-C bond networks after curing, making them difficult to recycle and leading to material waste and environmental pollution. In a study published in 2025 in Science by Professor Tao Xie and Researcher Ning Zheng’s team from Zhejiang University, a novel photopolymer chemical system based on dissociative network design was proposed. This system achieves closed-loop recycling of materials through the reversible photopolymerization and thermal dissociation of dithioacetal bonds while maintaining excellent mechanical properties. The core innovation of this work lies in the dynamic dissociative photochemical mechanism, providing a new approach for the design of high-performance recyclable photopolymers. |

Technical Principles and Design
1. The Core Chemical Mechanism of Dynamic Dissociative Networks
The irreversibility of traditional photopolymers stems from permanent cross-linked networks, while the core innovation of this study is the introduction of dithioacetal bonds as dynamic covalent cross-linking points. These bonds are formed through a click reaction between thiols (-SH) and bio-based vanillin aldehyde (-CHO) under the catalysis of a photogenerated acid (PAG) (see Figure 1A). The uniqueness lies in:
Light-triggered polymerization: Under 365 nm ultraviolet light (40 mW/cm²), PAG releases protons, activating the condensation reaction between thiols and aldehydes, generating dithioacetal bonds and forming a three-dimensional network (reaction time is only 20 seconds).Thermal-driven dissociation: Under acidic conditions (such as hydrochloric acid) at 80 °C, dithioacetal bonds can reversibly dissociate into thiols and aldehydes, generating low molecular weight oligomers. The dissociation process can be terminated through neutralization reactions (such as sodium bicarbonate), stabilizing the oligomer state, allowing it to be directly used for the next round of printing without purification.
Key Breakthroughs:
Closed-loop recycling: Traditional dynamic bonds (such as urethane bonds) require the addition of new monomers to replenish reactive groups, while this system directly generates photoreactive oligomers through dissociation, achieving a recycling efficiency of 100%.Modular main chain design: The molecular structure of the network main chain can be flexibly adjusted by varying the type and ratio of thiol cross-linkers (see Figure 1B). For example, using short-chain dithiol (such as ethylene glycol dithiol) can form a highly cross-linked rigid network; introducing long-chain polycaprolactone dithiol (molecular weight 6864.6) imparts semi-crystalline characteristics to the material (see Figure 3I).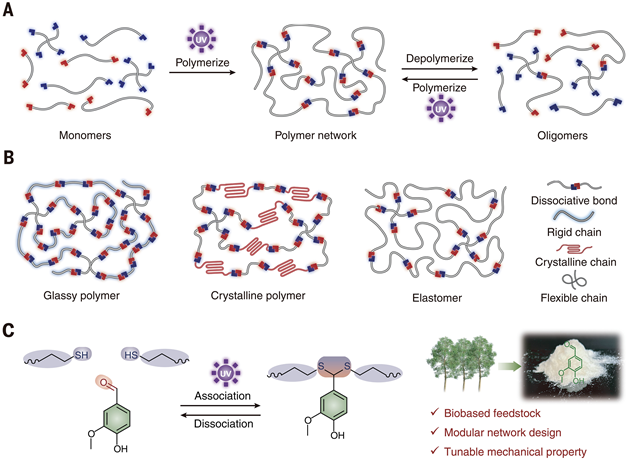 Figure 1. Design of Closed-loop Recyclable Photopolymer Networks
Figure 1. Design of Closed-loop Recyclable Photopolymer Networks
2. Chemical Reaction Kinetics and Stability
The research team systematically verified the feasibility of the chemical system using model compounds (reaction of vanillin with monothiol) (see Figure 2):
Photopolymerization kinetics: Under UV irradiation, the conversion rate of aldehydes increases linearly with time, reaching 93% within 20 seconds (see Figure 3C), meeting the rapid gelation requirements for 3D printing (gel point 86%).Thermal dissociation equilibrium: Under acidic conditions at 80 °C, dithioacetal bond dissociation follows a first-order kinetic model, reaching equilibrium within 4.5 hours (dissociation rate 31%). The dissociation state can be frozen through neutralization reactions to prevent reverse reactions, ensuring oligomer stability.Storage stability: Unactivated resin stored in the dark for 72 hours shows only a 0.5% consumption rate of aldehydes (see Figure 2A), indicating excellent storage performance and avoiding the pre-polymerization issues of traditional dynamic resins.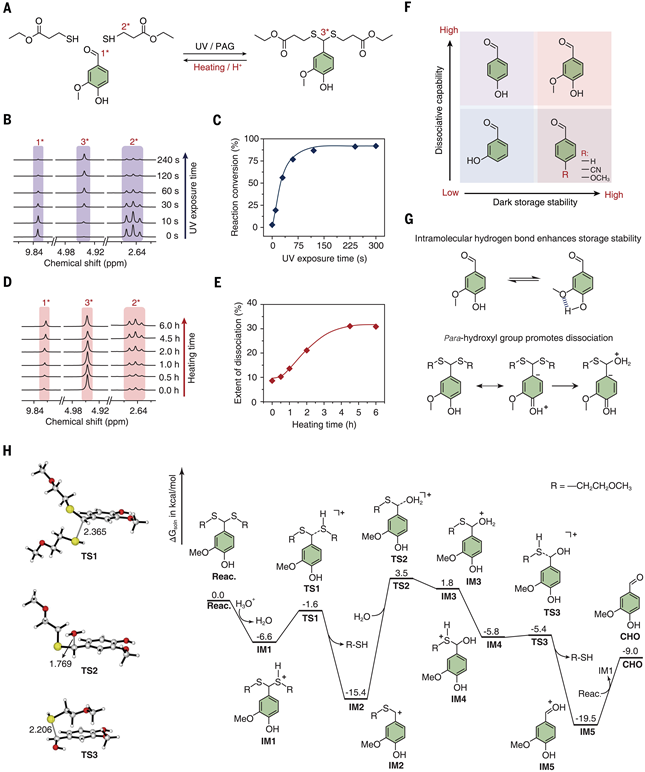 Figure 2. Study of Model Compounds in Dissociative Photochemistry.Molecular mechanism analysis: The molecular structure of vanillin (para-hydroxy and methoxy groups) reduces its acidity through intramolecular hydrogen bonding, inhibiting spontaneous condensation (see Figure 2G). During the dissociation process, cationic intermediates (IM2, IM3) are stabilized by the resonance effect of hydroxyl groups, promoting the cleavage of dithioacetal bonds (see Figure 2H). Density functional theory (DFT) calculations show that the activation energy for the dissociation reaction is 55.3 kJ/mol, which is highly consistent with experimental data.Material Properties and Characterization
Figure 2. Study of Model Compounds in Dissociative Photochemistry.Molecular mechanism analysis: The molecular structure of vanillin (para-hydroxy and methoxy groups) reduces its acidity through intramolecular hydrogen bonding, inhibiting spontaneous condensation (see Figure 2G). During the dissociation process, cationic intermediates (IM2, IM3) are stabilized by the resonance effect of hydroxyl groups, promoting the cleavage of dithioacetal bonds (see Figure 2H). Density functional theory (DFT) calculations show that the activation energy for the dissociation reaction is 55.3 kJ/mol, which is highly consistent with experimental data.Material Properties and Characterization
1. Modular Control of Mechanical Properties
By adjusting the molar ratio of tetrathiol cross-linkers and dithiol (DT-X system, where X is the proportion of tetrathiol), researchers achieved continuous performance tuning from elastomers to rigid materials (see Figure 3G-H):
Glass transition temperature (Tg): DT-0.2 (20% tetrathiol) has a Tg of 10°C, exhibiting elastomer properties (break elongation >1000%); DT-0.8 (80% tetrathiol) has a Tg of 56°C, with a modulus of 220 MPa, approaching the performance of engineering plastics.Semi-crystalline polymers: Introducing polycaprolactone dithiol into the network main chain results in a melting peak at 45°C (see Figure S12), with a modulus of 41.1 MPa and a break elongation of 1250%, combining flexibility and fatigue resistance (see Figure 3I).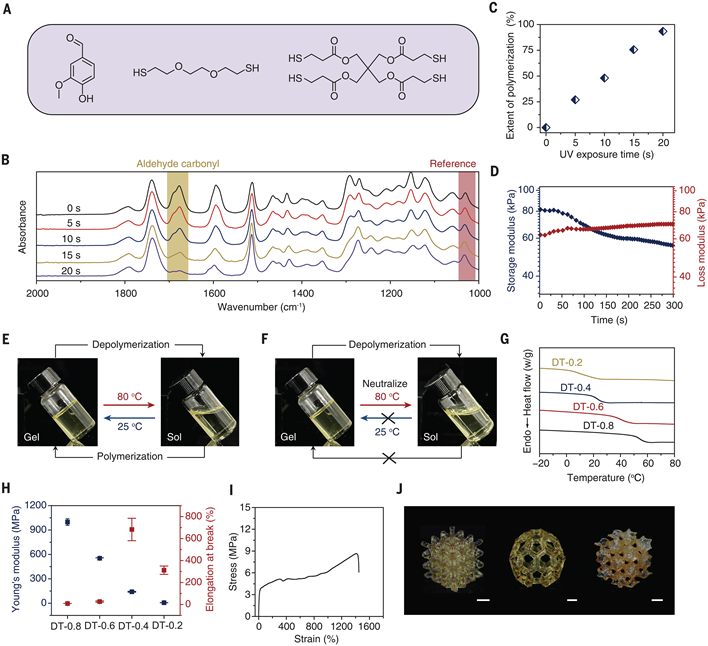 Figure 3. Synthesis and Characterization of Photopolymer Networks.
Figure 3. Synthesis and Characterization of Photopolymer Networks.
2. Closed-loop Recycling Validation and Durability
Taking DT-0.4 (modulus 141 MPa, break elongation 683%) as an example, cyclic experiments demonstrate that the material’s performance shows no significant degradation after multiple recycling (see Figure 4D):
Chemical consistency: Gel permeation chromatography (GPC) shows that the molecular weight distribution of the recycled products (Đ ≈ 1.2) is consistent with the initial oligomers (see Figure 4B); ¹H-NMR confirms that the ratio of thiols to aldehydes remains 2:1 (see Figure 4C).Mechanical stability: After 5 cycles of printing, the modulus and break elongation fluctuate by less than 5% (see Table S2), indicating that the network structure maintains integrity during the dissociation-recombination process.Environmental benefits: Life cycle assessment (LCA) shows that compared to traditional non-recyclable resins (literature 16), this process significantly optimizes 15 indicators, including greenhouse gas emissions (GWP reduced by 62%) and water resource consumption (WC reduced by 45%) (see Figure 4I).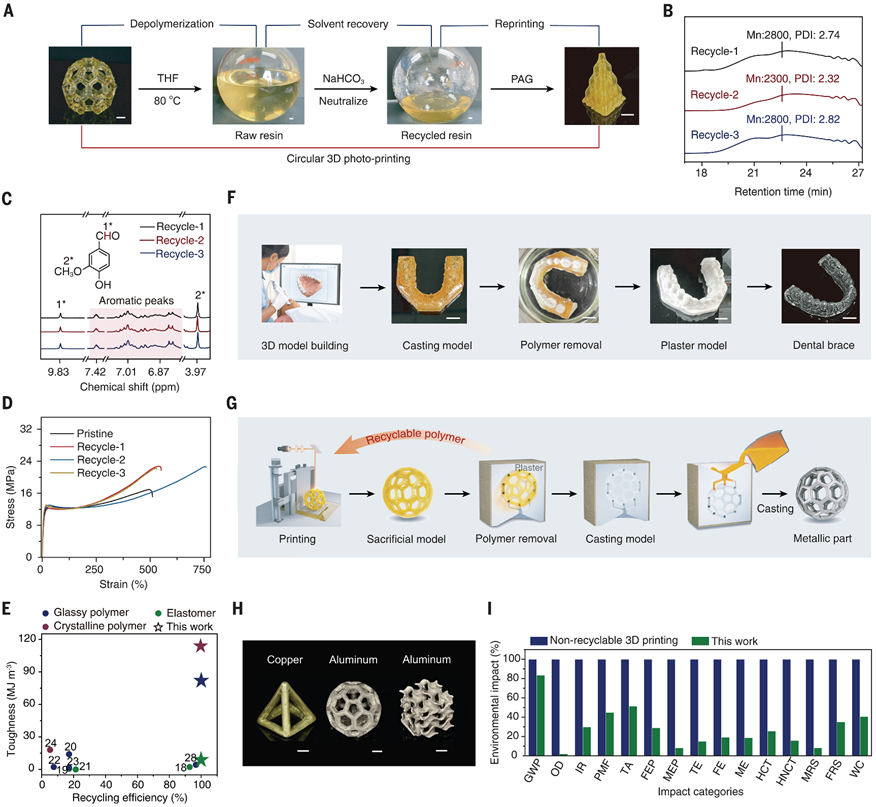 Figure 4. Cyclic 3D Printing and Its Applications.
Figure 4. Cyclic 3D Printing and Its Applications.
Practical Applications and Industrialization Potential
1. High-precision Manufacturing and Customization Applications
This photopolymer resin is compatible with commercial stereolithography equipment, achieving a printing resolution of 5 microns, suitable for the following scenarios:
Dental orthodontic molds: Printed sacrificial molds can be used to create plaster models, and after dissolving in hot water, the resin can be recovered and reused for new mold manufacturing (see Figure 4F). Compared to traditional thermoplastic molds (such as PLA), the surface finish of photopolymer molds is higher, with a detail restoration improved by 30%.Metal casting: By cyclically printing complex sacrificial molds (see Figure 4G), the same batch of resin can produce multiple batches of metal parts (see Figure 4H), reducing resin consumption by over 90%.
2. Technical Economic Comparison
Compared to traditional photopolymerization technologies, the advantages of this system include:
Cost savings: The resin recovery rate is 100%, and the cost per print is reduced by 70% (assuming 3 cycles of recycling).Process simplification: No solvents are required (vanillin can be directly dissolved in preheated thiols) and no post-curing steps (monomer residue <0.1%), shortening the production cycle by 50%.Environmental compliance: The use of bio-based vanillin and green solvents (such as 2-methyltetrahydrofuran) meets EU REACH regulatory requirements.
Conclusion and Future Prospects
This research successfully achieved closed-loop recycling of high-performance photopolymers through dynamic dissociative photochemistry design, with core contributions including:
Unity of full recovery and high performance: For the first time, 100% recovery efficiency and a modulus in the hundred MPa range are achieved in photopolymer resins.Freedom of molecular design: The modular network main chain supports the development of various materials such as elastomers and semi-crystalline polymers.Industrial feasibility: Compatible with existing photopolymerization equipment, and process parameters (such as exposure time and temperature) are easily scalable.
Future research directions:
Expanding the dynamic chemical toolbox: Exploring the photoreactivity of other dissociative dynamic bonds such as acetal and imine to broaden the applicability of materials.Optimization of dissociation conditions: Developing low-temperature dissociation catalysts (such as enzyme catalysis) to reduce energy consumption and increase dissociation rates.Integration of smart materials: Combining shape memory or self-healing functions to develop 4D printing applications.
This research not only provides an innovative paradigm for sustainable 3D printing, but its design concept of “dissociation-recombination” can also inspire the development of other high-performance recyclable materials (such as epoxy resins and polyurethanes), promoting the manufacturing industry towards a circular economy.
Original text: (Click the bottom left corner to read the original text)
Circular 3D printing of high-performance photopolymers through dissociative network design
Bo Yan, Tiantian Ni, Jingjun Wu, Zizheng Fang, Kexuan Yang, Ben H, Xingqun Pu, Guancong Chen, Chujun Ni, Di Chen, Qian Zhao, Wei Li, Sujing Li, Hao Li, Ning Zheng and Tao Xie
https://www.science.org/doi/10.1126/science.ads3880#sec-5