Source: YaoDu
Written by: -7℃ Edited by: Wanzi
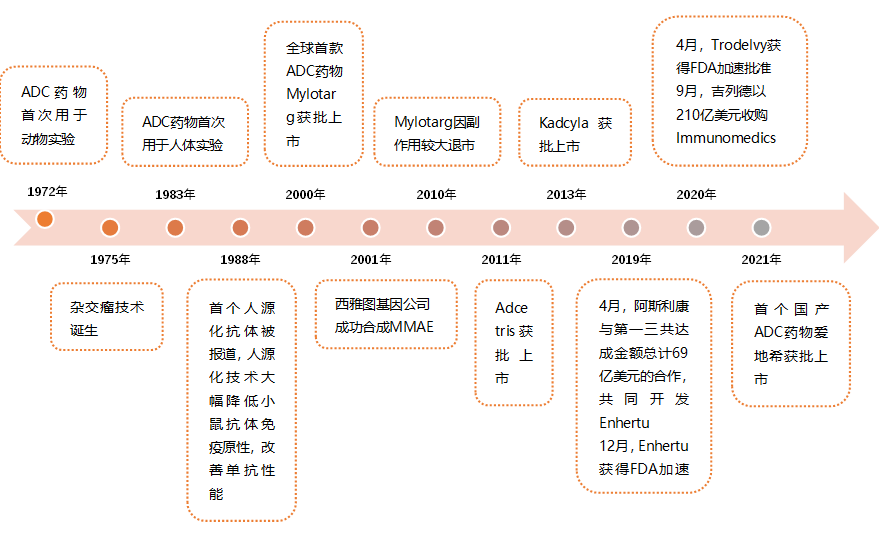
ADC (Antibody Drug Conjugate) is a complex formed by linking cytotoxic drugs to monoclonal antibodies targeting tumors, which combines the characteristics of targeted drugs and chemotherapy drugs, enabling precise treatment. The concept of ADC drugs was proposed as early as 1900, but it remained theoretical for a long time due to technological limitations. Between 1990 and 2000, monoclonal antibody drugs gained widespread clinical application, reducing the barriers to developing ADC drugs.
Figure 1: History of ADC Drug Development
ADC drugs have unique advantages
Broadening and Deepening Tumor Treatment
1Mechanism of Action of ADC Drugs
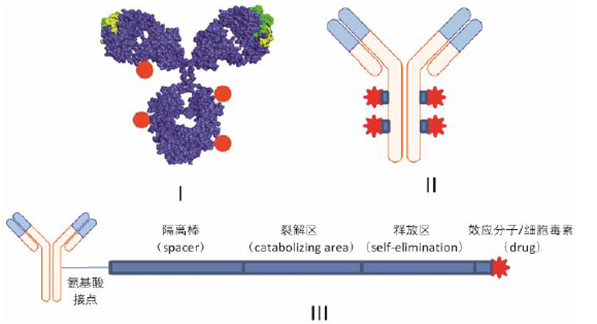
ADC is also known as “biological missiles”. It connects cytotoxic drugs similar to chemotherapy with monoclonal antibodies to achieve targeted killing of tumor tissues. ADC drugs consist of three components: antibody, linker, and effector molecule (small molecule cytotoxic drug).
Figure 2: Structure of ADC Drugs
The effector molecule is a toxic small molecule that directly kills tumor cells, which can be classified into DNA damaging types and microtubule inhibitor types based on their mechanisms. The linker is responsible for connecting the effector molecule with the monoclonal antibody and releasing it at the tumor tissue site. Depending on how it breaks, it can be categorized into cleavable and non-cleavable designs.
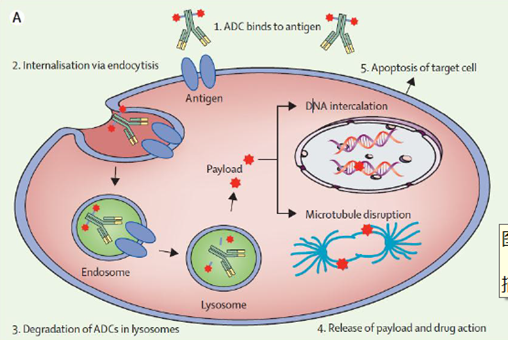
ADC drugs are injected into the bloodstream, where the antibody part specifically binds to antigens on the surface of tumor cells, and the resulting complex is engulfed by the cells. Inside the cells, cleavable linkers are sensitive to environmental factors within the tumor cells and can be cleaved by specific pH environments, proteases, or certain chemicals; ADC drugs with non-cleavable linkers are digested by lysosomes, releasing the drug. Some small molecules from ADC drugs can penetrate cell membranes and further kill surrounding cancer cells, known as the bystander killing effect. Additionally, ADCs also possess immune effects such as CDC, ADCC, and ADCP.
Figure 3: Mechanism of Action of ADC Drugs
2ADC Drugs Have Unique Advantages
ADC drugs combine the principles of targeted therapy and chemotherapy, thus possessing the advantages of both therapies. Since the antibody part can act directionally at the tumor site, ADCs have better safety compared to chemotherapy drugs, with fewer side effects. On the other hand, traditional targeted therapies work by inhibiting certain signaling pathways or physiological processes that promote tumor growth or killing, while ADCs primarily exert their effects by directly inhibiting tumor cell mitosis and damaging DNA structure.
Compared to targeted therapies such as monoclonal antibodies, ADCs expand their indications in three ways.
First, ADCs can target points not covered by targeted therapies. For example, the FIC drug Trodelvy has opened up therapies targeting Trop-2, providing new treatment options for advanced triple-negative breast cancer.
Second, for patients who have developed resistance to existing targeted therapies, ADC drugs can still be used to extend their response to treatment. For instance, Enhertu has shown good efficacy in patients resistant to trastuzumab.
Finally, compared to targeted therapies, some ADCs have lowered the requirements for target protein expression levels. Drugs like Enhertu and Trastuzumab have reduced the expression level requirements for HER2 molecules, which is expected to expand the indications for populations with low HER2 expression.
3High Barriers to Production and R&D of ADC Drugs
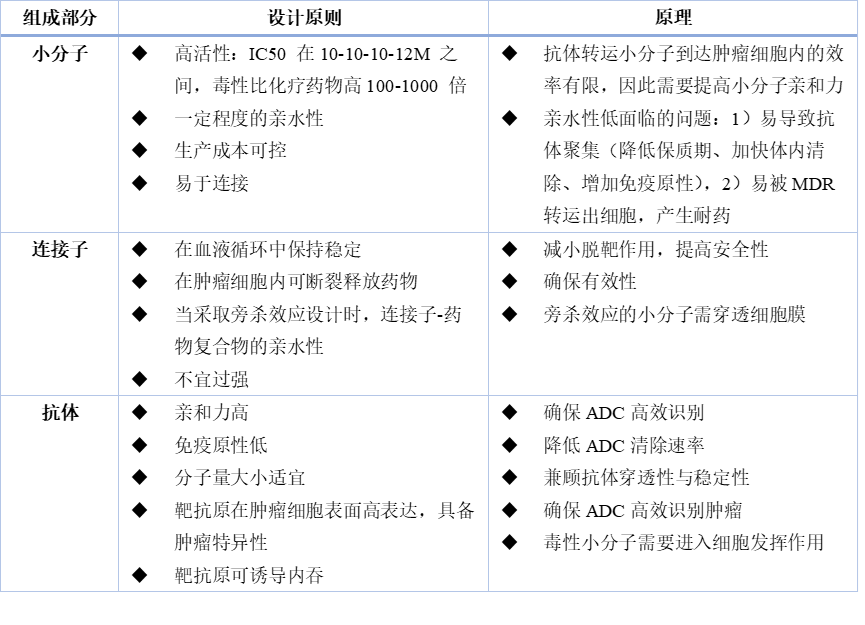
The technology platform is the primary barrier for ADC companies. The type of toxin, conjugation chemistry methods, and the stability of the linker all significantly impact the drug’s final clinical efficacy. For example, toxins that are too low in toxicity will limit the drug’s clinical effectiveness; excessively hydrophobic toxins can lead to aggregation of ADC molecules, increasing the difficulty of drug development; poor uniformity and controllability of conjugation reactions can result in excessive heterogeneity of ADC drugs, reducing clinical effectiveness; and linkers with poor stability can increase off-target toxicity, posing significant safety risks.
Table 1: Principles of ADC Design
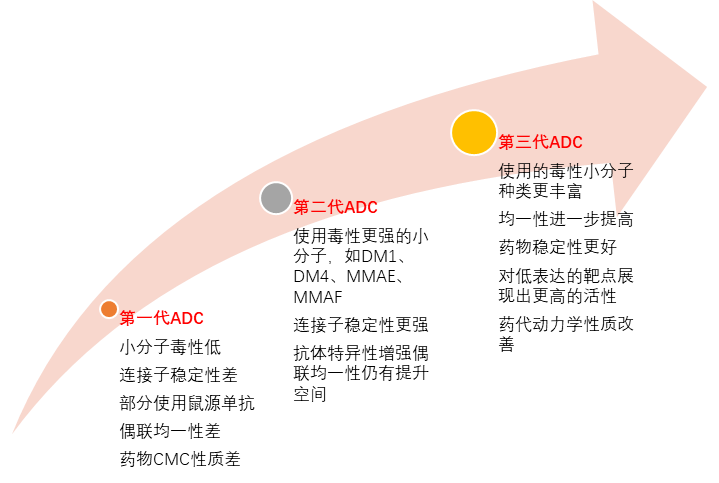
From the historical development of drugs, the iteration of technology platforms has also led to upgrades in the therapeutic effects and market competitiveness of ADC drugs. The first-generation ADC represented by Pfizer’s Mylotarg had high off-target toxicity and high drug heterogeneity, failing to achieve good sales returns. Second-generation ADCs based on Seagen and Immunogen technologies, such as Adcetris and Kadcyla, now have annual sales exceeding $1 billion. The drug Enhertu developed using the proprietary DXd platform by Daiichi Sankyo has achieved excellent clinical results and is expected to emerge as a new blockbuster in the future.
Figure 4: Characteristics of ADC Drugs Over the Years
4High Difficulty in CMC Process Development of ADC Drugs
Unlike monoclonal antibody drugs, ADC drugs need to combine biological macromolecules with highly active cytotoxic small molecules, which have significant differences in molecular weight, hydrophilicity, and other physical and chemical properties. In addition to requiring separate process development for the antibody and toxin, ADC drugs also require special process development for the conjugation reaction.
For example, when using thiol groups generated from reduced cysteine for conjugation, corresponding process development is needed to determine the optimal dosage of reducing agents, reaction temperature, and other factors. On the other hand, the physical and chemical properties of each component interact with the key quality attributes of the drug, making process development more constrained. For instance, the hydrophilicity of small molecule toxins and the amino acid sites for conjugation can affect the drug’s drug distribution and DAR value. (DAR (Drug to antibody ratio) refers to the number of small molecules attached to each antibody; as the DAR value increases, theoretically, the activity of a single ADC molecule is stronger.)
Due to the exposure risks associated with highly toxic drugs during ADC production, the manufacturing facilities require higher levels of protection. During production, the weighing of small molecule toxins must be done in isolators, and subsequent operations must take place in relatively negative pressure environments; the conjugation reactions of ADCs must be conducted in specially designed workshops, leading to higher capital investments than for general monoclonal antibody drugs.
Although developing an ADC drug requires enormous human, material, and financial resources, many pharmaceutical companies have been laying out R&D pipelines for ADC drugs in recent years. Currently, the number of ADC drugs approved on the market is still accelerating.
5Market Development of ADC Drugs
The world’s first ADC drug, Mylotarg, was approved in 2000. Poor uniformity and unstable pharmacological properties limited the efficacy of Mylotarg, leading to its market withdrawal in 2010 due to significant side effects.
From 2000 to 2010, no ADC drugs were approved globally. Companies like Seagen (formerly Seattle Genetics) spent years in R&D to yield the second-generation ADC drug represented by Adcetris.
From 2011 to 2018, a total of 4 ADC drugs were approved worldwide. In recent years, with the pharmaceutical industry continuously accumulating knowledge in target research, small molecule toxicity, and protein engineering, innovation and development in the ADC field have accelerated. Since 2019, there has been a surge in ADC drug approvals, with a total of 9 drugs launched, exceeding the total of the previous nearly two decades.
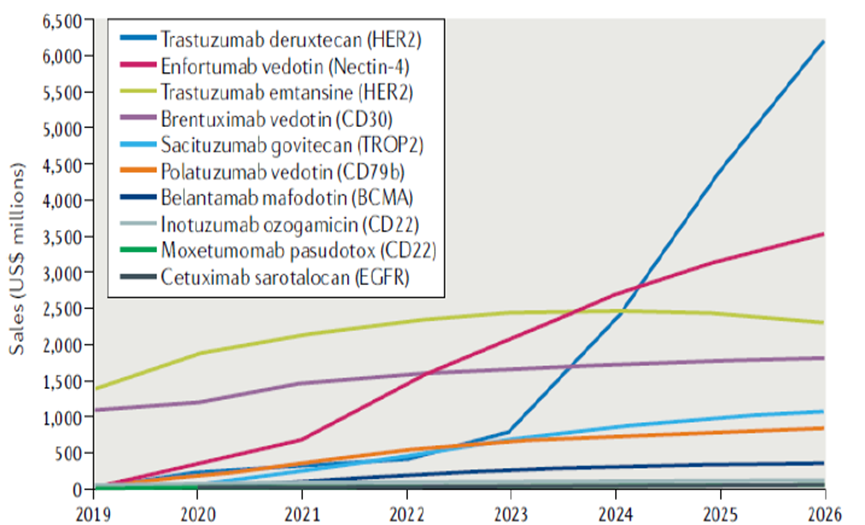
Benefiting from the gradually expanding indications and excellent efficacy of ADC drugs, sales have repeatedly reached new highs. In 2021, global ADC sales were nearly $5 billion, with a compound annual growth rate of about 30% from 2014 to 2021. Adcetris was approved in 2011, with global sales of approximately $1.27 billion in 2021. Kadcyla was approved in 2013, with global sales of approximately $2.13 billion in 2021. New generation ADC drugs have immense growth potential, with Enhertu and Padcev both receiving FDA approval in 2019, with sales of $500 million and $340 million in 2021, respectively. Nature estimates that these two drugs will reach sales of $6.2 billion and $3.5 billion by 2026.
Figure 5: Historical Sales Scale of ADC Drugs
Figure 6: Sales Forecast for ADC Drugs
Summary
According to statistics, there are currently over 400 ADC drugs under research globally, with more than 200 entering clinical stages. In China, there are over 170 ADC drugs under research, with nearly 60 entering clinical stages. From the approved and researched drugs, tumor indications dominate, accounting for about 449, or approximately 96% of all ADC drugs. However, the occurrence and development of tumors is a highly heterogeneous process, making it difficult for a single product to cover all treatment scenarios and clinical needs. Therefore, each product needs to find its positioning based on its characteristics to cover the clinical needs it is best suited for, thus forming a differentiated competitive strategy. The future development of ADC drugs will continue to be monitored by YaoDu.
References:
[1] Journal of Pharmacy
[2] Nature Reviews
[3] Company Annual Reports
Registration for August Live Lecture by ZhiYao Research Society