
Click the blue text above to follow us

Introduction
The lithium-ion battery capacitor utilizes a dual-material positive electrode and capacitor materials, combining the characteristics of lithium-ion batteries and supercapacitors, filling the gap in meeting the demands for high power and energy density applications. However, research on the working mechanism of dual-material positive electrodes in lithium-ion battery capacitors is still in its infancy, particularly the lack of kinetic analysis. The distribution of relaxation times method allows for effective analysis of kinetic characteristics by deconvoluting electrochemical impedance spectra. This study is the first to apply this method to lithium-ion battery capacitors, systematically analyzing the relaxation times and impedances of various electrochemical processes involving activated carbon, LiNi1/3Mn1/3Co1/3O2, and dual-material cathodes with different charge states. The polarization kinetics of the dual-material cathode reveal the synergistic effects of the two materials. The ohmic impedance remains stable, while the interface impedance and charge transfer impedance decrease with increasing voltage, and the diffusion impedance first decreases and then increases.In situ electrochemical impedance spectra further indicate that the addition of activated carbon enhances the rate performance of the dual-material cathode, and increasing temperature accelerates the reaction kinetics of the dual-material cathode. Additionally, dynamic analysis of the soft carbon anode and full battery shows good compatibility between the dual-material positive and negative electrodes. These findings enhance the understanding of dual-material cathode kinetics and guide the development of high-performance and safe lithium-ion battery capacitors.

01

Research Background
With the rapid development of new energy vehicles, electronic products, and industrial fields, the requirements for electrochemical energy storage devices (EESD) in practical applications are continuously increasing. The most common EESDs include lithium-ion batteries (LIB) and supercapacitors. LIBs rely on the intercalation mechanism of lithium ions (Li) for energy storage, offering advantages such as high energy density and low self-discharge rate, but perform poorly in scenarios requiring high power and rapid charging. In contrast, supercapacitors store energy through the adsorption/desorption of ions at the double electric layer, characterized by high power density and long cycle life, but their low energy density limits their applications. Therefore, there is an urgent need to develop an EESD that combines high specific energy and high specific power to meet various practical demands. The lithium-ion battery capacitor (LIBC) is a hybrid system that connects LIBs and supercapacitors in parallel at the electrode level, typically consisting of a dual-material cathode containing battery and capacitor materials and a battery material anode. By combining lithium-ion intercalation/deintercalation reactions with the rapid physical adsorption/desorption reactions of the double electric layer, LIBCs integrate the advantages of both systems, exhibiting excellent electrochemical performance.

02

Overview of the Full Text
Steady-state battery systems produce corresponding response signals under small amplitude external disturbance input signals, and the response signals output by different electrochemical processes exhibit differences in relaxation times. By extracting time scale information, the physical and chemical processes of the battery can be effectively analyzed. Alternating current electrochemical impedance spectroscopy (EIS) is a high-precision and non-destructive advanced characterization method that is crucial for studying the kinetics and surface phenomena of electrochemical batteries. However, it is challenging to directly identify different electrochemical processes from EIS results and extract the impedance of each process, and it is often supplemented by equivalent circuit model (ECM) methods to obtain parameters with different physical meanings.
This work tested the impedance spectra of various active material electrodes and systematically analyzed their kinetic processes using the DRT method, including AC, NCM, soft carbon (SC), and hybrid dual-material electrodes. In situ EIS experiments were designed and employed to obtain impedance data during charging and discharging processes, visualizing the changes in the physical and chemical processes of various electrodes. The effects of rate and temperature on the kinetics of the hybrid cathode were studied using in situ EIS testing. Finally, a LIBC with SC electrode as the anode was assembled and analyzed using the DRT method. These detailed analyses provide clear explanations for various dynamic processes, corresponding time scales, and polarization impedances in LIBCs, offering valuable insights for further development.

03

Illustrated Guide
The complete process of DRT analysis is illustrated in Figure 1. For half-cells, the current density is based on the active mass of the working electrode, while for full cells, the current density is based on the active mass of the cathode. For non-in situ EIS testing, the potential is easier to control and achieve compared to the state of charge (SOC).
Figure 1. Schematic diagram of the DRT analysis process.
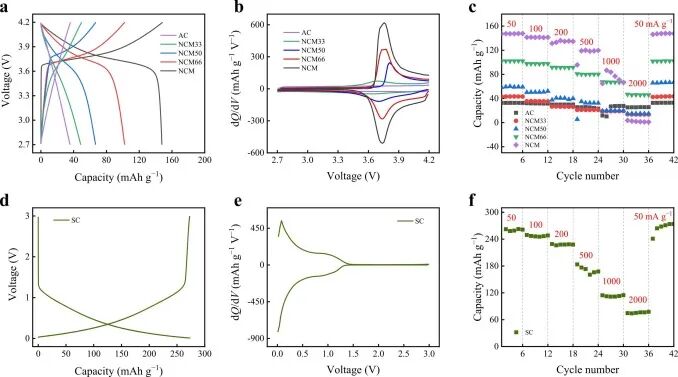
Figure 2. (a, b, c) Charging/discharging voltage curves of different cathode half-cells and (d, e, f) SC half-cells, dQ/dV curves and rate capabilities.
The normalized DRT results of different cathode half-cells at different voltages are shown in Figure 3. Each DRT curve is divided into several characteristic peaks with different relaxation times τ, where the number of peaks represents the number of electrochemical processes, and the peak area reflects the impedance of the corresponding electrochemical process.
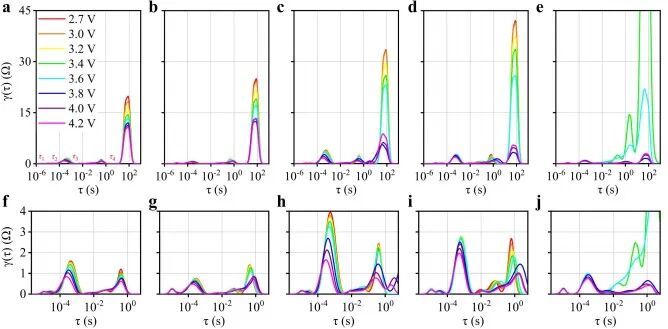
Figure 3. (a) AC, (b) NCM33, (c) NCM50, (d) NCM66, and (e) NCM half-cells’ DRT curves at different voltages; (f) AC, (g) NCM33, (h) NCM50, (i) NCM66, and (j) NCM half-cells’ partially expanded DRT curves.
Figure 3f-j shows the partial enlarged view of the normalized DRT results of τ set to 6 s from 2 × 10−6, and the peaks of RCT and Ri for all voltages are recorded in Figure 4. The maximum values of both RCT and Ri for all cathodes show a decreasing trend with increasing voltage, indicating that polarization gradually decreases during charging. Notably, the τ values of RCT and Ri of NCM33 are significantly smaller than those of the other two hybrid cathodes, reflecting the rapid response rate of the adsorption/desorption reactions on the AC material.
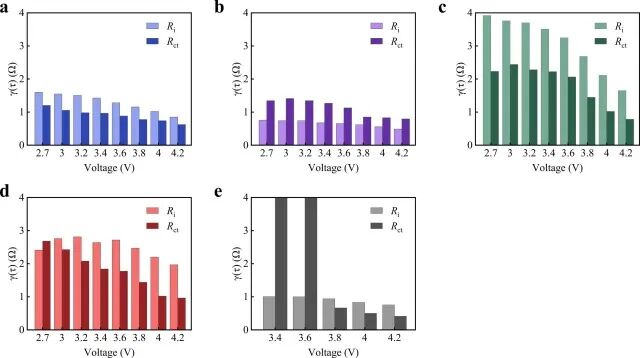
Figure 4. Maximum values of RCT and Ri for (a) AC, (b) NCM33, (c) NCM50, (d) NCM66, and (e) NCM half-cells at different voltages.
As shown in Figure 5, a DRT schematic diagram based on SOC and voltage was constructed, where the voltage corresponding to SOC is displayed as the second y-axis in the SOC-based DRT schematic diagram. To represent more effectively, the impedance in the DRT schematic diagram is normalized, and the impedance values are reflected through a color hierarchy.
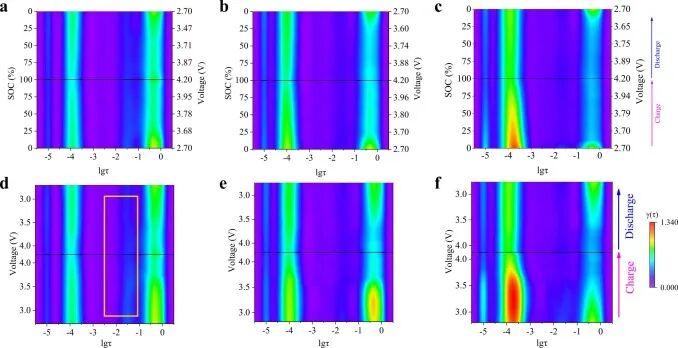
Figure 5. (a, d) DRT schematic diagrams based on SOC and voltage for NCM33, (b, e) NCM50, and (c, f) NCM66 half-cells.
Figure 6 records the SOC-based DRT schematic diagram, which is calculated based on the in situ EIS results of three hybrid cathode half-cells at room temperature, 40°C, and 60°C at approximately 0.2 C. For comparison, the impedances of the same half-cell at different temperatures are normalized to reflect the impedance values through a color hierarchy. The polarization impedances of all three hybrid cathode half-cells show a decreasing trend with increasing temperature, which can be attributed to the high temperature promoting charge transfer and ion diffusion.
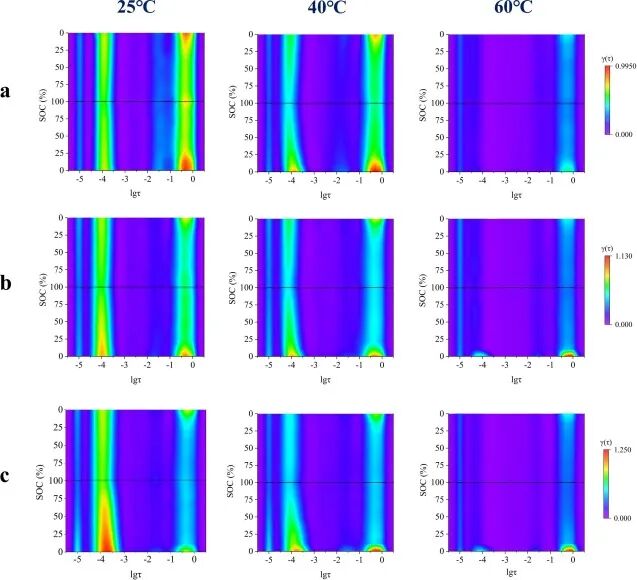
Figure 6. SOC-based DRT schematic diagrams for (a) NCM33, (b) NCM50, and (c) NCM66 half-cells at different temperatures.
Within the voltage range of 1.0 −0.01 V, the EIS results of SC//Li soft-pack half-cells at different open circuit voltages (OCV) (Figure 7a) indicate that further DRT analysis has good quality, as shown in Figure 7b. Figure 7c illustrates the τ range of different electrochemical processes of the SC electrode. Figure 7d records the maximum values of RCT and Ri at different OCVs, where Ri shows a trend of first increasing and then decreasing during the lithium-ion intercalation process, while RCT shows the opposite trend.
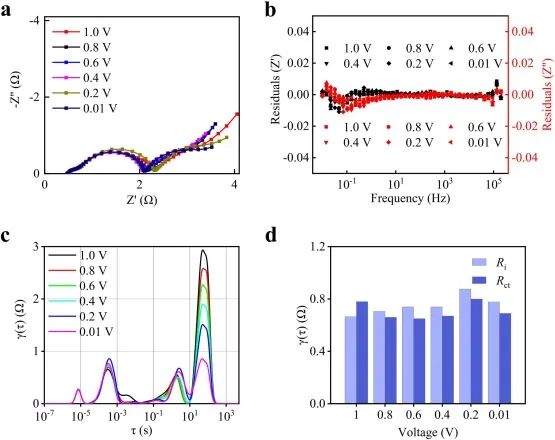
Figure 7. SC//Li soft-pack half-cell: (a) EIS results, (b) relative KK residuals at different voltages; (c) DRT profiles, and (d) maximum values of RCT and Ri at different voltages.
The partial DRT results of the full battery, NCM50, and SC half-cells are shown in Figure 8a. Figure 8b displays the relationship curve between constant current charging/discharging voltage and SOC at 0.2 C and the SOC-based DRT schematic diagram of the full battery. Additionally, the EIS of the full battery at different OCVs was tested, and the corresponding DRT results are recorded in Figure 8c for analyzing the diffusion process.
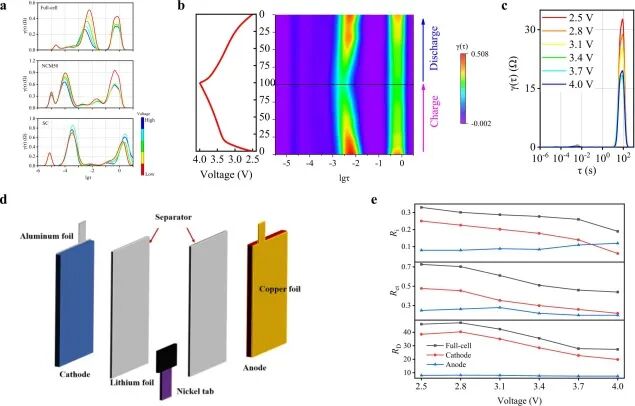
Figure 8. (a) Comparison of partial DRT results of the full battery, NCM50, and SC half-cells; (b) relationship curve between constant current charging/discharging voltage and SOC at 0.2 C and SOC-based DRT schematic diagram of the full battery; (c) DRT curves of the full battery at different open circuit voltages; (d) schematic diagram of the three-electrode bag testing structure; (e) maximum values of Ri, RCT, and RD for the full battery, cathode, and anode within the three-electrode bag testing structure at different voltages.

04

Article Summary
In this work, the polarization kinetics of lithium-ion battery capacitors and the improvement mechanisms of battery/capacitor materials on hybrid cathode performance were systematically studied by extracting time scale information through the deconvolution of Kramers-Kronig validated EIS data using the distribution of relaxation times method.
- • The relaxation distribution functions of AC, NCM, and hybrid dual-material electrodes exhibit four distinct characteristic peaks, ordered by relaxation time as follows: contact peak, interface peak, charge transfer peak, and diffusion peak. The ohmic and contact processes of various electrodes are independent of the state of charge, maintaining stable impedance, while the charge transfer and interface impedances gradually decrease with increasing voltage, indicating reduced polarization. The synergistic effects of AC and NCM materials lead to composite characteristics in the diffusion process of the hybrid cathode. The hybrid cathode transfers energy through a rapid adsorption/desorption mechanism in the low voltage range, thereby reducing diffusion impedance. During the plateau period, as the intercalation reaction dominates, overcoming the energy barrier of the battery material, the diffusion impedance significantly decreases. With further increases in voltage, the reduction of the concentration gradient hinders the diffusion process, leading to an increase in characteristic peaks. Notably, hybrid cathodes with higher AC content show a smaller increase.
- • In situ EIS tests designed and tested to obtain multidimensional dynamic information indicate that the addition of AC material can alleviate the increase in charge transfer impedance of the hybrid cathode at high rates. Furthermore, the interface impedance and charge transfer impedance of the hybrid cathode decrease with increasing temperature, accompanied by a shortening of the interface impedance relaxation time.
- • Finally, SC electrodes and full batteries were also prepared and analyzed using DRT, indicating good dynamic compatibility between the hybrid cathode and anode.

05

How to Join the Group

Group Chat: AI Machine Learning Research Group
How to join: Scan the code to join the group or add the editor’s WeChat: wu2014640669
Please note: Unit-Name-Research Direction (otherwise, it will not be approved), the editor will review and invite you to join the group.
[Original Link]
https://www.sciencedirect.com/science/article/pii/S2949822824003605#sec0070
Disclaimer: This article aims to convey and share research information for personal learning, reference, and academic exchange purposes only, not for commercial use. All cited references in the text have indicated the authors and sources. All images appearing in this article are reprints, and due to limited capabilities, there may be inaccuracies in interpretation. If there are issues related to intellectual property protection or others, please contact the email [email protected], and we will coordinate to handle it as soon as possible.