
 Image source: J. Am. Chem. Soc.Introduction:Professor Ruben Martin reported a photoinduced palladium-catalyzed interceptive decarboxylative allylation reaction. This strategy innovatively achieves atom pair exchange and a series of skeletal contraction and extension reactions, providing a non-traditional retrosynthetic approach for complex molecule construction, while offering a modular and versatile synthetic tool for rapidly and efficiently obtaining sp3 hybridized skeletal structures in drug discovery.
Image source: J. Am. Chem. Soc.Introduction:Professor Ruben Martin reported a photoinduced palladium-catalyzed interceptive decarboxylative allylation reaction. This strategy innovatively achieves atom pair exchange and a series of skeletal contraction and extension reactions, providing a non-traditional retrosynthetic approach for complex molecule construction, while offering a modular and versatile synthetic tool for rapidly and efficiently obtaining sp3 hybridized skeletal structures in drug discovery.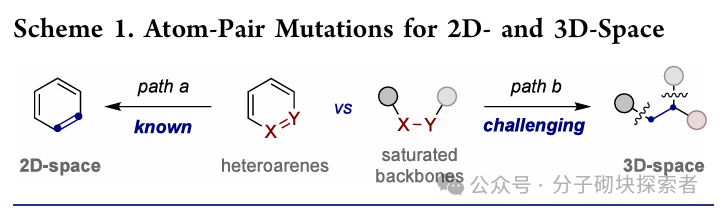
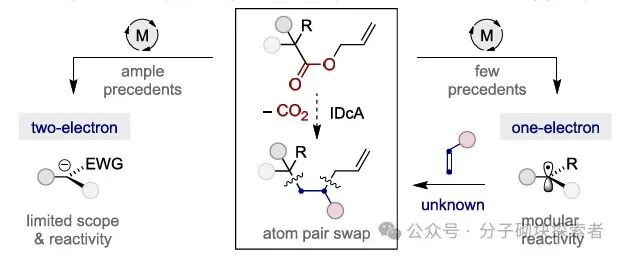
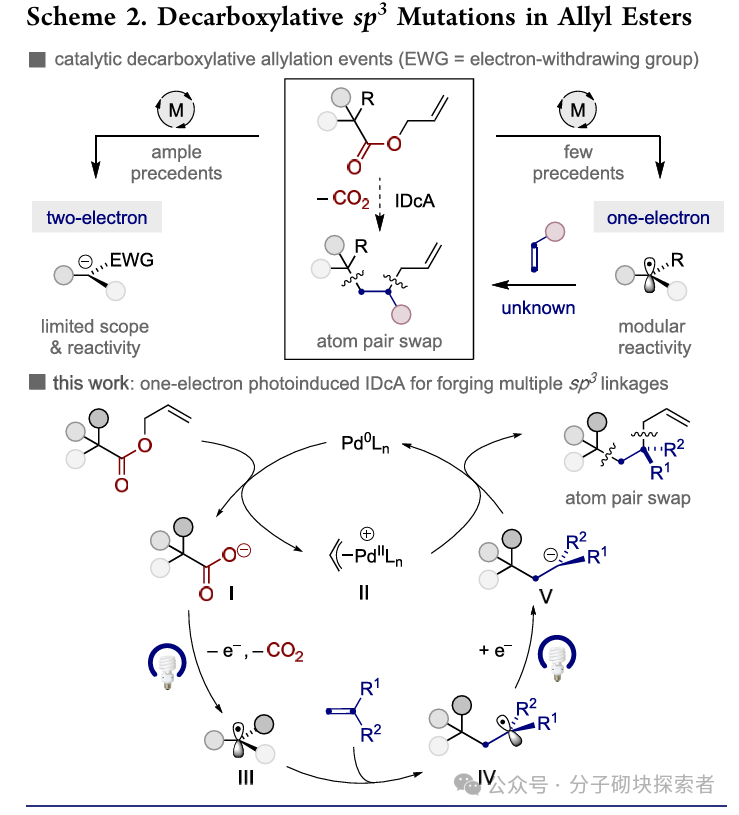 Image source: J. Am. Chem. Soc.In organic synthesis, how to rapidly and efficiently construct molecular complexity from simple precursors has always been a core challenge. Among them, one of the most attractive research directions is to develop techniques that can precisely modify molecular frameworks through atom deletion, insertion, or exchange, achieving predictable site selectivity. Although significant progress has been made in atom pair mutation of heteroaromatic systems (path a), the atom pair mutation for constructing sp3 hybridized fragments still mainly relies on stoichiometric oxidants, cyclic amines, or ring strain release strategies. Given that the increase in the number of sp3 hybridized carbon atoms can significantly improve the clinical success rate-related characteristics of drug molecules, developing a catalytic, versatile transformation method to convert simple acyclic saturated structures into sp3 frameworks may become an important strategy in drug discovery.
Image source: J. Am. Chem. Soc.In organic synthesis, how to rapidly and efficiently construct molecular complexity from simple precursors has always been a core challenge. Among them, one of the most attractive research directions is to develop techniques that can precisely modify molecular frameworks through atom deletion, insertion, or exchange, achieving predictable site selectivity. Although significant progress has been made in atom pair mutation of heteroaromatic systems (path a), the atom pair mutation for constructing sp3 hybridized fragments still mainly relies on stoichiometric oxidants, cyclic amines, or ring strain release strategies. Given that the increase in the number of sp3 hybridized carbon atoms can significantly improve the clinical success rate-related characteristics of drug molecules, developing a catalytic, versatile transformation method to convert simple acyclic saturated structures into sp3 frameworks may become an important strategy in drug discovery.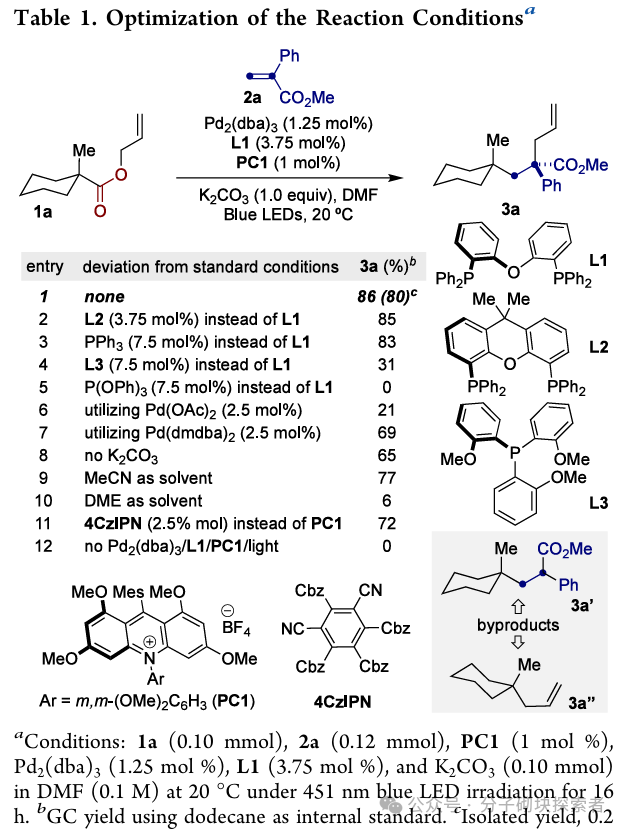 Image source: J. Am. Chem. Soc.
Image source: J. Am. Chem. Soc.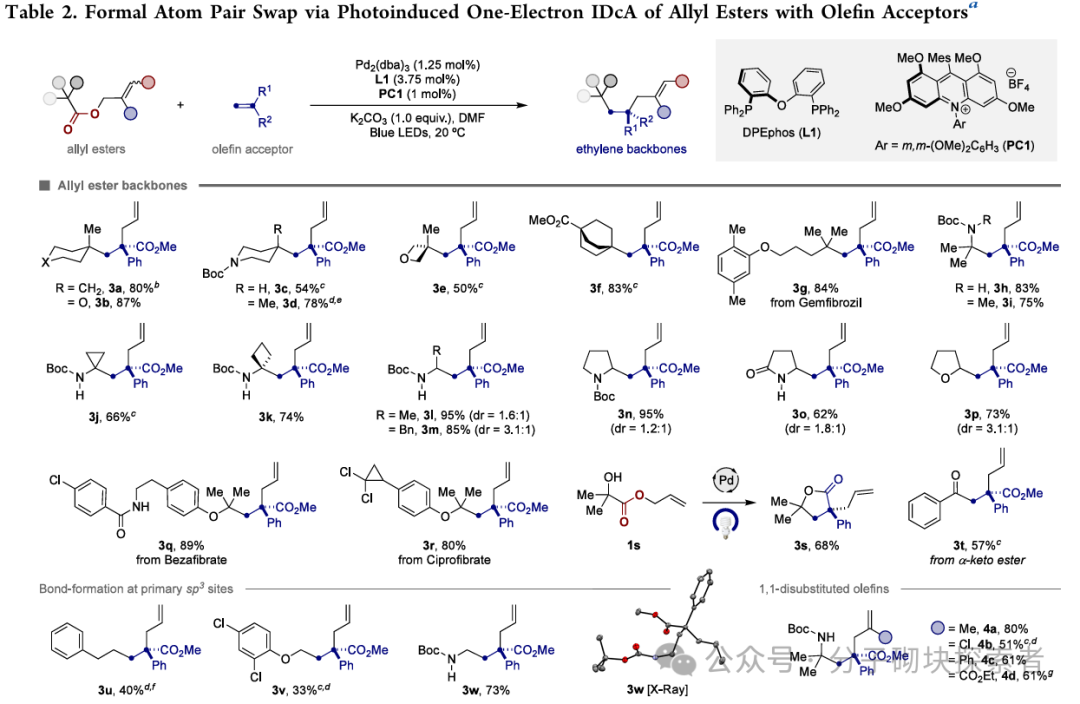
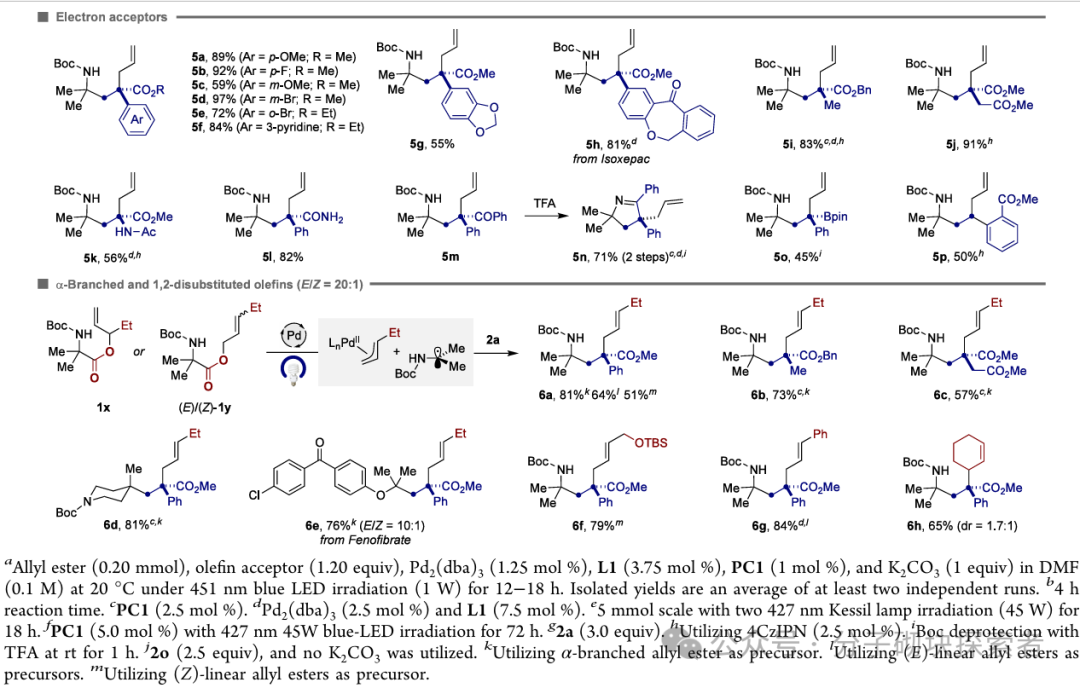 Image source: J. Am. Chem. Soc.
Image source: J. Am. Chem. Soc.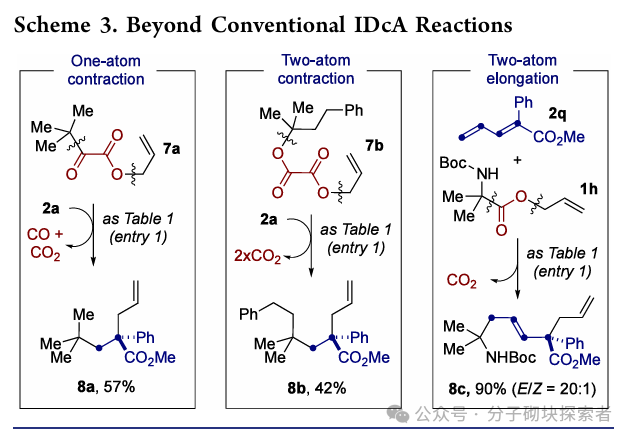
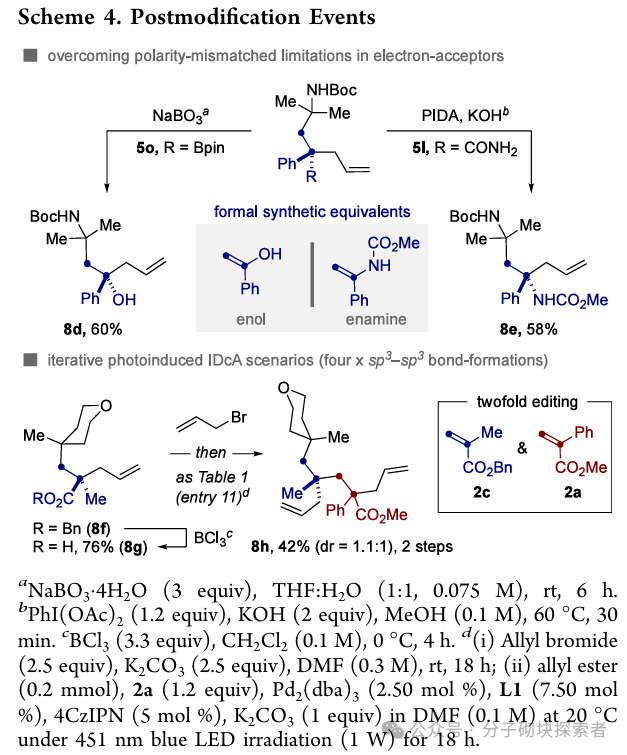
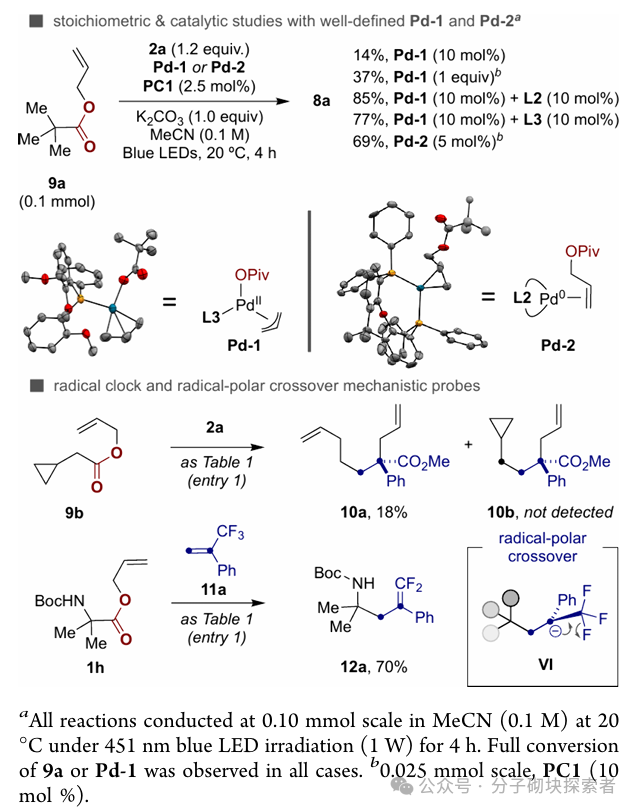 Image source: J. Am. Chem. Soc.Conclusion:A novel palladium-catalyzed photoinduced single-electron decarboxylative allylation (IDcA) reaction system has been developed, suitable for simple acyclic allyl ester compounds. This method features mild reaction conditions and a wide substrate applicability, enabling the realization of formal atom pair exchange or a series ofcontraction and extension of skeletal atoms. This strategy not only expands the application scope of decarboxylative allylation reactions but also provides a new pathway for the rapid and efficient construction of drug chemistry-related molecular frameworks through unconventional bond cleavage methods.References:
Image source: J. Am. Chem. Soc.Conclusion:A novel palladium-catalyzed photoinduced single-electron decarboxylative allylation (IDcA) reaction system has been developed, suitable for simple acyclic allyl ester compounds. This method features mild reaction conditions and a wide substrate applicability, enabling the realization of formal atom pair exchange or a series ofcontraction and extension of skeletal atoms. This strategy not only expands the application scope of decarboxylative allylation reactions but also provides a new pathway for the rapid and efficient construction of drug chemistry-related molecular frameworks through unconventional bond cleavage methods.References:
Pd-Catalyzed Photoinduced Interceptive Decarboxylative Allylation
J. Am. Chem. Soc. 2025
https://pubs.acs.org/doi/10.1021/jacs.5c03044