Since the emergence of several new drugs for the treatment of solid tumors, such as larotrectinib and entrectinib, the concept of “broad-spectrum anti-cancer drugs” has officially entered public awareness. Years later, more and more broad-spectrum anti-cancer drugs have moved from the laboratory to clinical settings, officially ushering in this precise yet “broad-spectrum” era of cancer treatment.
HER2 is also a target with broad-spectrum anti-cancer potential. Currently, some drugs targeting this receptor have initiated clinical trials with the aim of broad-spectrum anti-cancer.
One representative, the antibody-drug conjugate (ADC) trastuzumab deruxtecan (DS-8201, Enhertu), recently received breakthrough therapy designation from the FDA, marking a significant step towards the era of “precision treatment” for HER2 solid tumors!
Multiple solid tumors show sustained relief for over 22 months, initial potential of new therapies
On September 1, 2023, Enhertu (trastuzumab deruxtecan) received two new breakthrough therapy designations from the FDA, one for adult patients with unresectable or metastatic HER2-positive (IHC3+) solid tumors who have progressed after prior treatment and have no alternative treatment options, and the other for treating HER2-positive (IHC 3+) metastatic colorectal cancer patients who have previously received two or more treatment regimens.
According to data presented at the 2023 ASCO conference, trastuzumab deruxtecan achieved an overall response rate of 37.1% for treating multiple solid tumors; the median duration of response was 11.8 months, with the longest duration in IHC 3+ patients reaching 22.1 months. The trial participants included patients with various solid tumors, including cervical cancer, endometrial cancer, ovarian cancer, cholangiocarcinoma, pancreatic cancer, bladder cancer, and more.
Additionally, early results from the DESTINY-CRC01 trial showed that trastuzumab deruxtecan had an overall response rate of 45.3% for treating colorectal cancer.
Multiple new drugs expand indications, covering over 12 types of cancer
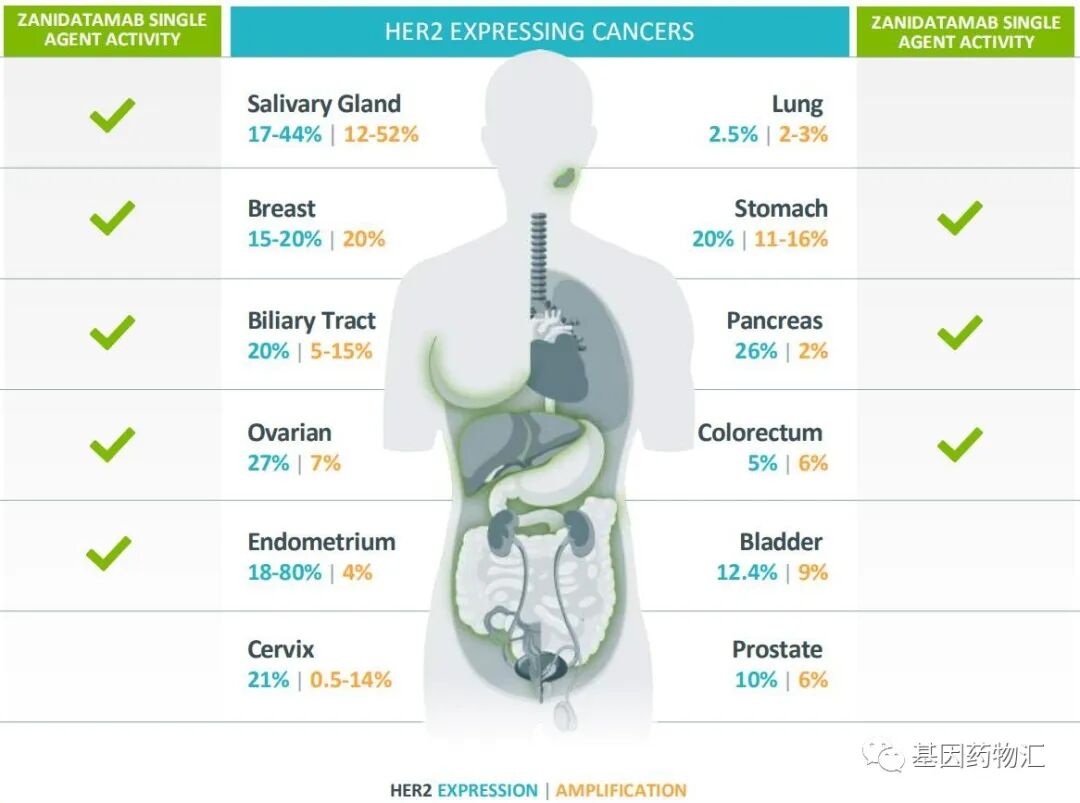
As mentioned at the beginning of this article, HER2 is also a target with broad-spectrum anti-cancer potential. The detection rate of high HER2 expression in lung cancer is approximately 2.5%, in breast cancer it is about 15%~25%, in gastric cancer it is around 20%, in cholangiocarcinoma it is about 20%, in ovarian cancer it is approximately 27%, and in endometrial cancer it can reach 18%~80%.
Regarding the efficacy of new drugs, as previously mentioned, trastuzumab deruxtecan achieved an overall response rate of 37.1% for patients with multiple solid tumors; additionally, the new drug SHR-A1811 achieved an overall response rate of 61.6%, and DB-1303 achieved an overall response rate of 44.2% for treating multiple solid tumors, demonstrating excellent performance.
Currently, there are many ADC new drugs and trial projects targeting HER2-positive malignancies, including several products from China, some of which are third-generation products with potential comparable to the second-generation ADC trastuzumab deruxtecan. Many of these projects are currently recruiting patients, and interested individuals can refer to the methods in the poster at the end of the article to submit their medical records and apply to participate in clinical trials.
Of course, in addition to ADCs, there are many new treatment options targeting HER2 in clinical research.
For instance, the CAR-T therapy targeting HER2 has already shown significant benefits for patients. One cholangiocarcinoma patient achieved clinical remission after just one cycle of CAR-HER2-T cell therapy, with one metastatic lesion completely disappearing.
The CAR-M cell (CAR-macrophage) therapy CT-0508 has also received FDA fast track designation as a potential treatment option for solid tumor patients. Like T cells, macrophages are naturally occurring immune cells in the body. However, macrophages function across the body’s non-specific immune processes (humoral immunity) and specific immune processes (cellular immunity), capable of engulfing and digesting dead cells, cellular debris, or other pathogens, while also activating other immune cells by secreting specific substances (cytokines) and presenting antigens analyzed during digestion to T cells, promoting the process of cellular immunity.
Based on B-cell cancer vaccines, there is also promising potential. Current data shows that the B lymphocyte vaccine HER2-Vaxx, used in combination with chemotherapy for treating HER2-positive gastric cancer, can reduce the risk of death by 41.5% compared to chemotherapy alone. The longest survival time for treated patients has already exceeded 2.5 years!
With the advancement of medicine, more and more new drugs are entering clinical practice, striving to surpass the efficacy of current medications and innovate in many unprecedented fields. We eagerly anticipate the future development of these drugs and hope that this vigorous progress can translate into tangible improvements in patients’ survival and quality of life.
*This content is for reference only and should not be used as a basis for medication. Please choose treatment options under the guidance of a medical professional.