 Continuing from the previous article, this article will continue to introduce the types of free radicals.The Ohio State UniversityNagibproject team summarized the polarities of halogen, sulfur, silicon, and boron free radicals, as well as nitrogen free radicals.See the table below for halogen, sulfur, silicon, and boron free radicals.6。Nagibproject team noted that some effects are also cumulative. For example, in the α-Cl series, more chlorine substituents lead to higher electrophilicity (mono-substituted < di-substituted < tri-substituted:1.1, 1.3, 1.5 eV).This effect was also verified in the α-F series, where fluorine also increased electrophilicity (mono-substituted < di-substituted < tri-substituted:1.1, 1.2, 1.7 eV).However, similar to other heteroatoms, the opposing electronic effect of halogen substituents (resonance donor but inductive acceptor) results in α-F free radicals being less electrophilic than β-F free radicals (inductive effect only), with a significant difference (1.1 vs 1.5 eV).The distal β-Cl free radical is also more electrophilic than the α-Cl free radical (1.6 vs 1.1 eV).Mixed halogen or carbonyl and halogen substitutions produce progressively more electrophilic free radicals, such asCl5- acetone (2.6 eV) andCl2- acetonitrile (2.1 eV), both of which are more electrophilic than perfluorobutyl free radicals (1.9 eV).
Continuing from the previous article, this article will continue to introduce the types of free radicals.The Ohio State UniversityNagibproject team summarized the polarities of halogen, sulfur, silicon, and boron free radicals, as well as nitrogen free radicals.See the table below for halogen, sulfur, silicon, and boron free radicals.6。Nagibproject team noted that some effects are also cumulative. For example, in the α-Cl series, more chlorine substituents lead to higher electrophilicity (mono-substituted < di-substituted < tri-substituted:1.1, 1.3, 1.5 eV).This effect was also verified in the α-F series, where fluorine also increased electrophilicity (mono-substituted < di-substituted < tri-substituted:1.1, 1.2, 1.7 eV).However, similar to other heteroatoms, the opposing electronic effect of halogen substituents (resonance donor but inductive acceptor) results in α-F free radicals being less electrophilic than β-F free radicals (inductive effect only), with a significant difference (1.1 vs 1.5 eV).The distal β-Cl free radical is also more electrophilic than the α-Cl free radical (1.6 vs 1.1 eV).Mixed halogen or carbonyl and halogen substitutions produce progressively more electrophilic free radicals, such asCl5- acetone (2.6 eV) andCl2- acetonitrile (2.1 eV), both of which are more electrophilic than perfluorobutyl free radicals (1.9 eV).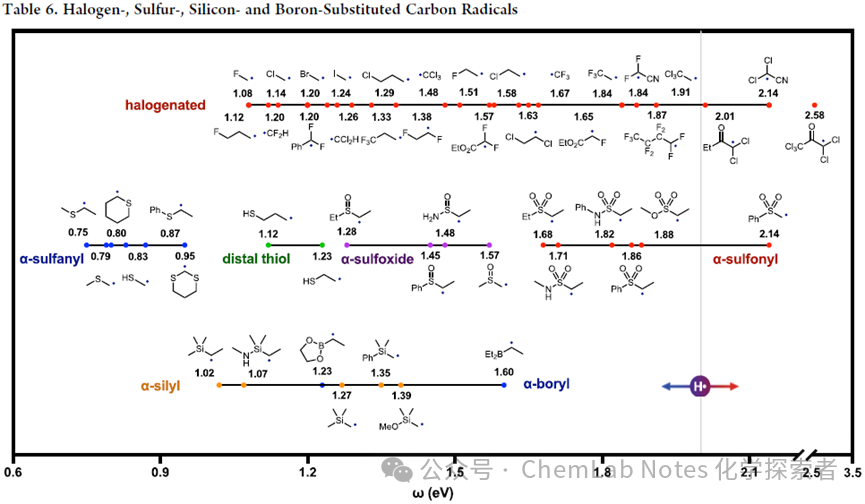
The impact of different substituents on the electronic properties of free radicals is summarized in the above figure, highlighting the roles of resonance and inductive effects.
-
Resonance vs Induction
α-F substituents: increase nucleophilicity through resonance electron donation.
Other halogens: Larger orbitals are unsuitable for resonance mixing, leading to stronger electrophilicity through inductive effects. The order of electrophilicity is:I > Br > Cl > F.
Multiple substitutions: Di- and tri-substitutions amplify these effects.
2. Sulfur:
Sulfur vs oxygen: More polarizable sulfur increases electrophilicity compared to oxygen.
α-SH and α-SMe free radicals: are more electrophilic than α-OH or α-OMe free radicals (0.8 eV vs 0.7 eV).
Distal thiol vs ether induction: Thiol induction effects (1.1−1.2 eV) exceed ether effects (1.0−1.1 eV).
3. Sulfonyl group::
α– sulfoxide free radical (S=O): has lower electrophilicity (1.3 eV), compared to α– carbonyl(C=O, 1.6 eV).
α– sulfonyl free radical (S(=O)2): has stronger electrophilicity (1.7−2.1 eV), suitable for kinetically challenging carbon–hydrogen atom transfer (HAT), unlike the less electrophilic α- carbonyl free radicals.
4. Silicon/boron:
Trend: Increased electrophilicity due to poor orbital mixing and stronger inductive effects (compared to resonance effects), similar to Cl > F pattern.
α– silicon-based and α– boron-based free radicals: are more electrophilic (1.0−1.6 eV), more so than α- sulfur free radicals (0.8 eV), which may explain the practicality of α– silicon-based free radicals in remote HAT.
Nitrogen free radicals see table7。These open-shell intermediates provide valuable tools for forming biologically relevantC−N bonds. Considering that nitrogen (3.0) has a higher electronegativity (χ) than carbon (2.6), and ω is proportional to χ², it is not difficult to understand that most nitrogen-centered free radicals are more electrophilic than the aforementioned carbon-centered free radicals. Notably, in this table, the scale guide (ω:H ∼ 2) is now centered, rather than being on the right as in previous tables. As expected, electrophilicity increases with the addition of neighboring protecting groups. For example, in the amine series (red), ω for theseNH-R free radicals increases:−Me,−Ph,−P(O),−NO2,−CN(ω:1.4, 1.7, 2.1, 2.2, 2.5 eV)。Interestingly, this wide range contrasts the nucleophilic amine free radicals with electrophilic amide and imine free radicals (or electron-withdrawing substituted amines:−CN,−N2)。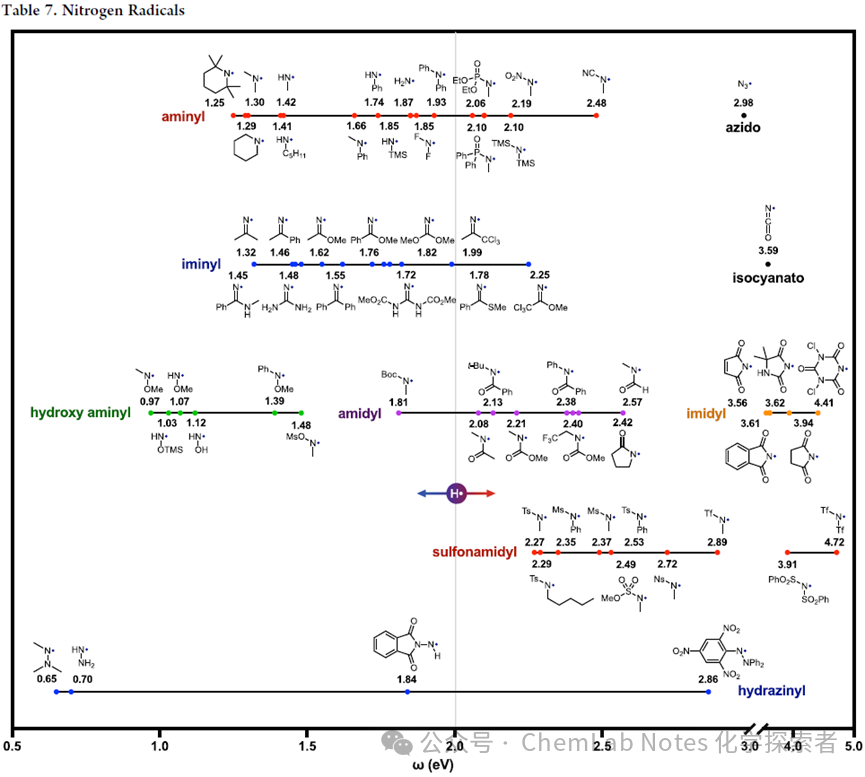 In the above figure, the impact of different substituents on the electronic properties of free radicals is summarized:
In the above figure, the impact of different substituents on the electronic properties of free radicals is summarized:
1.Orbital hybridization: The right shift of the imine series (blue) and azide (black) relative to simpleN- alkyl amine indicates an increase in electrophilicity with the increase ofs- character (sp3 < sp2 < sp), as seen in alkyl, vinyl, and alkynyl free radicals.
2.α– heteroatom effects: Further exploration ofN- substituents, hydroxyl amine (green) and hydrazine (blue) free radicals’ α– oxygen electron-releasing lone pairs replicate the α effect observed in peroxyl and hydroxylamine free radicals, leading to more nucleophilic free radicals than typical amines (<1.1 vs 1.4 eV).
3.Protecting groups: Conversely, electron-withdrawing groups increase electrophilicity, with the trend as follows: amine< carbamate < amide (purple) < sulfonamide (red) < imide (i.e., diamide; orange) < disulfonamide (red). These electron-withdrawing groups are strong enough to overcome the nucleophilic α– heteroatom effects, as seen in the case of hydrazine (0.7 eV) being replaced by phthalimide (1.8 eV) or trinitrobenzene (2.9 eV).
4.Hydrogen atom transfer (HAT): Notably, most nitrogen-centered free radicals used for selective HAT are more electrophilic than H• (center). That is, disulfonamide (4 eV) and amide (2 eV) are often used for intermolecular HAT. However, less nucleophilic imines, imine esters, or monosulfonamides (>1.4 eV) also frequently appear in intramolecular HAT.
This article is quite long, and we will continue to discuss different types of free radicals in detail over the next few days. Interested friends can like, follow, and bookmark.
References:
https://doi.org/10.1021/jacs.4c06774
J. Am. Chem. Soc. 2024, 146, 28034−28059
Note: All images in this article are sourced from the reference. If there are any copyright issues, please contact the author in a timely manner for proper handling. If you have any questions about this literature, feel free to communicate and discuss with the editor.