

Acute myeloid leukemia (AML) has a generally poor prognosis, with a 5-year survival rate of approximately 27%. Some subgroup studies indicate that patients with treatment-related AML (t-AML), older AML patients (aged ≥60 years), relapsed/refractory AML (R/R-AML) patients, and those who develop AML from other hematological diseases have worse prognostic outcomes. In high-risk AML patients, the high-dose cytarabine and mitoxantrone (HiDAC / mito) regimen is effective and well-tolerated compared to standard-dose cytarabine combined with an anthracycline regimen.
Abnormal epigenetics have been confirmed to be associated with the pathogenesis of AML, including abnormal DNA methylation. In treatment-naive AML patients, the complete remission (CR) rate for single-agent DNA methyltransferase inhibitors azacitidine (AZA) or decitabine ranges from 7% to 25%. Currently, researchers believe that part of the clinical activity of these drugs is mediated through the regulation of epigenetics related to important genes associated with myeloid leukemia.
This article will introduce a phase 1 clinical trial that evaluated the efficacy of AZA combined with the HiDAC / mito regimen in high-risk AML patients. The primary objective of the study was to determine the recommended dose of AZA for the phase 2 study. The secondary objectives were to assess the safety, response rate, and survival of the regimen.
Key Points
The recommended dose of AZA in the phase 2 study is 75 mg/m2 daily from days 1 to 5, followed by HiDAC / mito treatment on days 6 and 10.

Patients aged ≥60 years with treatment-naive or treatment-related AML, as well as those with NPM1 or IDH2 mutations, showed a high response rate.
Study Methods
The study population included high-risk AML patients aged ≥18 years with an ECOG score of 0-2. High-risk patients in this trial included t-AML, R/R-AML, or treatment-naive AML patients aged ≥60 years, MDS-AML, acute phase myeloproliferative diseases, and CMML-AML. The genetic risk stratification for the study was determined according to the 2010 ELN risk stratification scheme. Patients with acute promyelocytic leukemia, those who underwent major surgery, those receiving concurrent cancer treatment (except hydroxyurea), or those who participated in other clinical trials within the past 2 weeks were excluded. The clinical characteristics of the enrolled patients are shown in the table below.

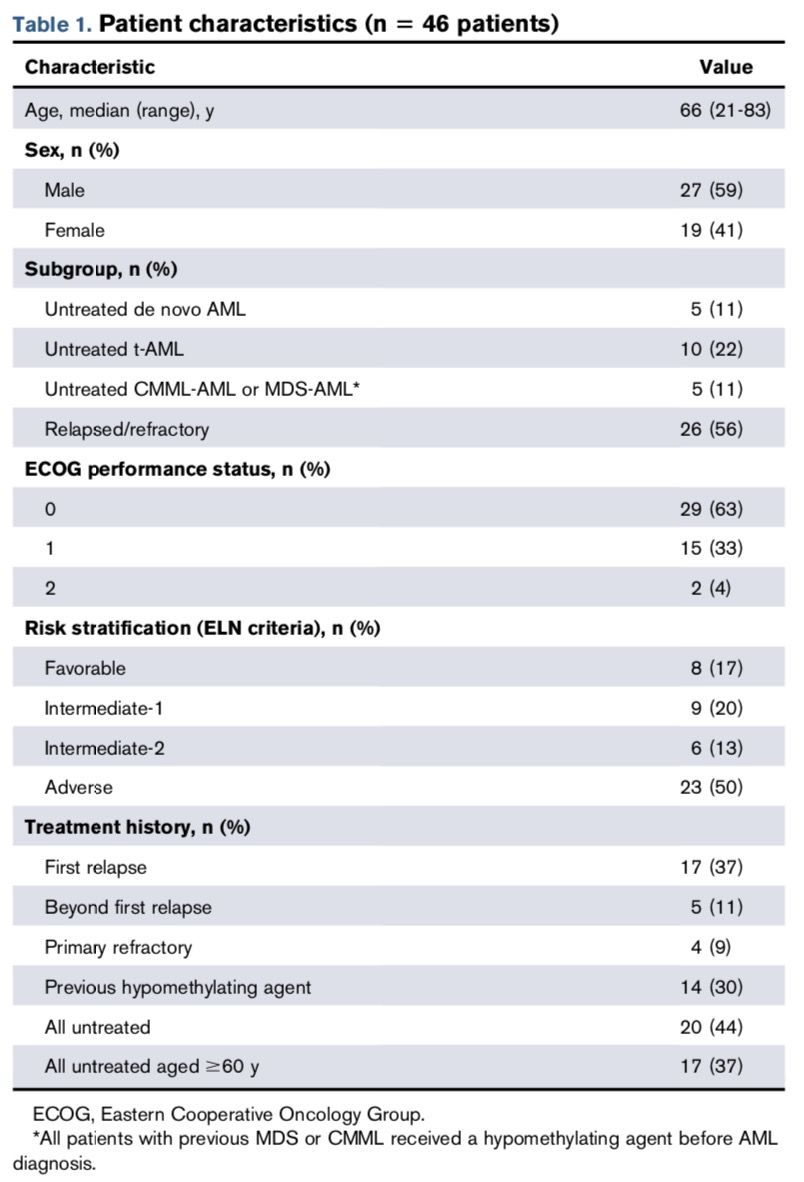
A total of 46 patients were enrolled in the study, with a median age of 66 years. Pre-treatment with demethylating agents to alter epigenetics can improve response rates in high-risk AML patients (including those with relapsed/refractory disease). Therefore, in this study, azacitidine (AZA) was administered prior to high-dose cytarabine (HiDAC) and mitoxantrone (mito). Patients received AZA at doses of 37.5, 50, or 75 mg/m2 subcutaneously or intravenously once daily from days 1 to 5; on days 6 and 10, they received HiDAC (3000 mg/m2) and mito (30 mg/m2) (the HiDAC/mito dose was reduced by 33% in elderly subjects).
The trial design employed a 3+3 dose escalation scheme, where AZA doses were divided into three levels: 37.5 mg/m2, 50 mg/m2, and 75 mg/m2. Three patients were treated at the lower dose level of AZA, and if the dose was tolerable (0 DLT events in 3 patients, or <2 DLT events in 6 patients), further patients could be enrolled at that dose level while proceeding to the next higher dose trial. Thus, each dose level had 12 to 18 subjects.

Dose-limiting toxicity (DLT) was defined as grade 4 or higher non-hematological toxicity events (excluding nausea/vomiting lasting ≤48 hours or liver function abnormalities lasting ≤48 hours), grade 3 non-hematological toxicity lasting ≥7 days, or persistent bone marrow suppression lasting ≥56 days. Toxicity events and adverse events were recorded and graded according to the Common Terminology Criteria for Adverse Events (CTCAE) version 4.0 from the National Cancer Institute.
Study Results01 Efficacy Analysis
Among all patients enrolled in the study, 41% (19/46) achieved CR, and the overall response rate (ORR) (CR+CRi) was 61% (28/46). There was no significant difference in response rates between the AZA doses (P=0.69). For treatment-naive patients aged ≥60 years, the ORR was 76% (13/17). Among these treatment-naive elderly patients, those with t-AML (8/8 [100%]) and treatment-naive AML (4/5 [80%]) responded better to the drug compared to CMML and MDS-AML patients (P=0.019).
According to the ELN risk stratification, all 8 low-risk patients responded to treatment, while only 9 out of 23 high-risk patients had a response. Among the 8 low-risk patients, five had NPM1 mutations but no FLT3-ITD mutations. The ORR (CR+CRi) for patients who had previously received demethylating agents was 36% (5/14), while for those who had not, the ORR (CR+CRi) was 72% (23/32) (P=0.047). Specific data is shown in the table below.

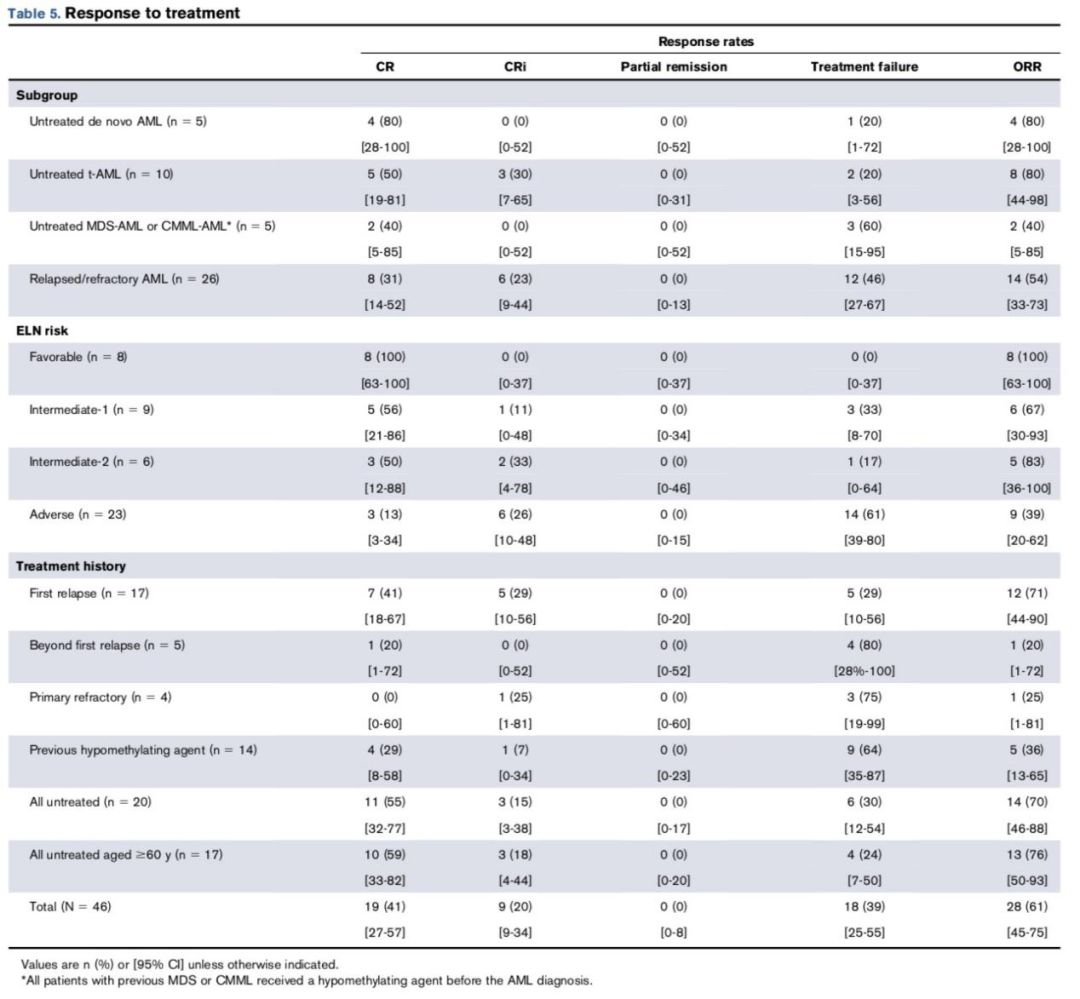
02 Safety Analysis
During the trial, a total of 28 grade 3-4 non-hematological toxicity events occurred. No grade 5 toxicity events were reported. Two DLT events occurred (both in the same patient): acute liver failure and acute kidney injury confirmed by biopsy due to venous embolism. This patient was a 64-year-old female with relapsed t-AML who had previously received chemotherapy and radiotherapy for breast cancer and mantle cell lymphoma. The data from the table also indicates that the recommended AZA dose for the phase 2 trial is 75 mg/m2.
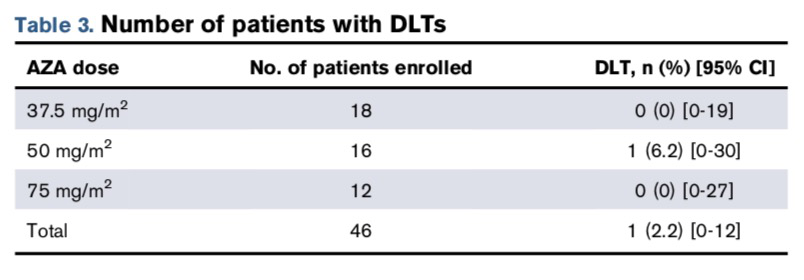
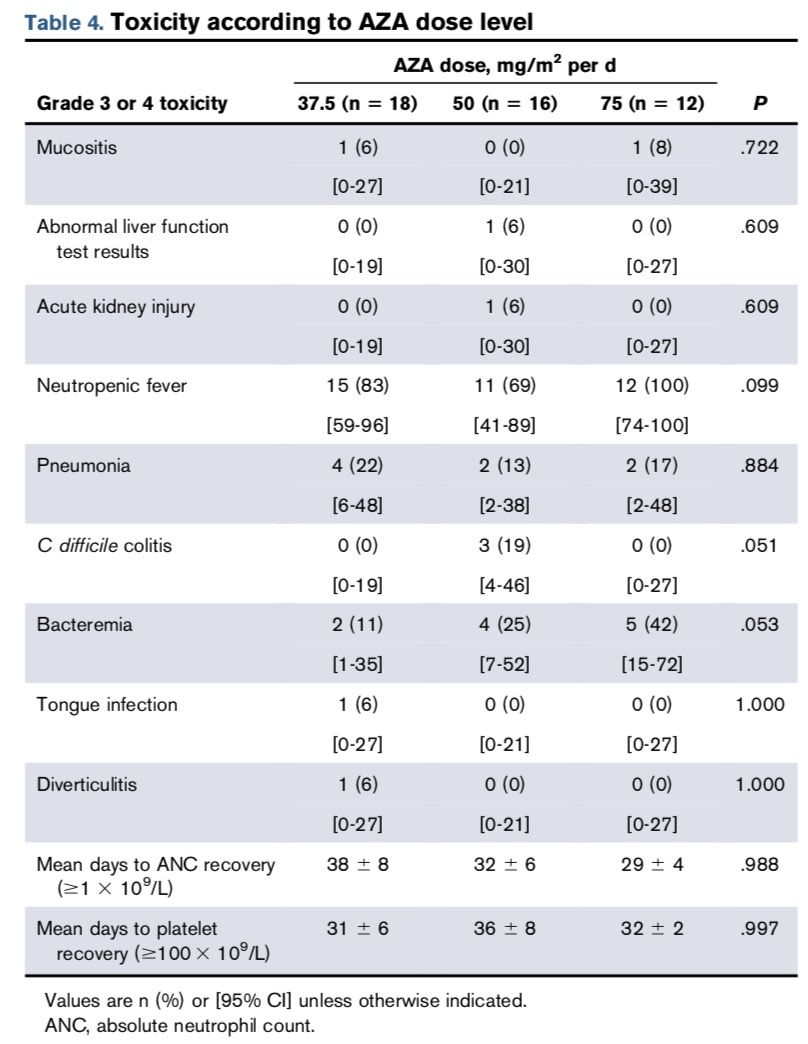
Neutropenia was the most common toxic reaction, occurring in 38 patients (83%), with 2 cases being grade 4 toxicity events, while the rest were grade 3. However, these were not DLT events, as hematological toxicities such as neutropenia often accompany AML treatment. Specific data is shown in the table below.

Conclusion
Compared to treatment-naive patients with MDS-AML and CMML-AML, patients aged ≥60 years with treatment-naive AML, treatment-related AML, and de novo AML showed better responses to the drug. Patients classified as low-risk by ELN (P=0.008), those with NPM1 mutations (P=0.007), or IDH2 mutations (P=0.03) generally had good responses to the drug, while those with TP53 mutations (P=0.03) had poorer responses. Additionally, this phase 1 clinical trial demonstrated that AZA at a dose of 75 mg/m2 daily for 5 days, followed by HiDAC combined with mitoxantrone treatment, is a safe and effective regimen for high-risk AML patients. Although there were no significant differences in efficacy and toxicity among the various AZA dose levels in this study, the recommended dose for the phase 2 trial was set at 75 mg/m2 with the aim of exploring the maximum dose.
References: Kirk E. Cahill, Yasmin H. Karimi, Theodore G. Karrison, et al. A phase 1 study of azacitidine with high-dose cytarabine and mitoxantrone in high-risk acute myeloid leukemia. Blood Adv 2020; 4 (4): 599–606.

 Click “Read the original text” to progress together.
Click “Read the original text” to progress together.