
First Author:Wang Lingxiao(Lingxiao Wang)Corresponding Author:Wu Yuen(Yuen Wu)、Yu Zhenqiang(Zhen-Qiang Yu)、Huang Hao(Hao Huang)Affiliation:University of Science and Technology of China, Shenzhen University, National University of Defense TechnologyPaperDOI:10.1002/anie.202506663
 Overview
Overview
Single-atom catalysts (SACs) have attracted significant attention in the field of CO2 electroreduction due to their high atomic utilization and unique electronic structure. However, the regulation mechanism of higher coordination shells (beyond the second shell) on catalytic performance remains unclear. This study uses nickel tetraphenylporphyrin (NiTPP) as a precursor and selectively cleaves the C-C single bonds at the β-carbon sites through a mild pyrolysis strategy, preserving the first and second coordination shells of the Ni-N4 active center while eliminating the peripheral π-electron delocalization effect. The optimized catalyst (NiTPP@CNT-450) achieves a Faradaic efficiency (FECO) of 85.9% at -1.4 V vs RHE (a 29-fold increase compared to the original catalyst) and maintains 98.3% CO selectivity at an industrial-level current density (500 mA cm-2). Combined experimental and theoretical calculations reveal that the elimination of long-range π-electron delocalization significantly enriches the electron density at the Ni sites, reducing the formation energy barrier of the *COOH intermediate, thereby accelerating the reaction kinetics. This study provides a new paradigm for optimizing catalytic performance through higher coordination shell engineering.
 Background
Background
The performance of single-atom catalysts is determined by the local coordination environment of the active sites (first and second shells), but the long-range electronic delocalization of higher coordination shells (> second shell) has long been overlooked in its role in regulating reaction barriers. For example, the long-range interactions of FeN4 sites can optimize the adsorption energy of *OH, while the higher coordination shell nitrogen doping at Ru centers can enhance propylene desorption. However, traditional synthesis methods struggle to precisely control the long-range coordination environment, leaving the mechanisms of higher shells unclear. How to retain the proximal coordination structure of the metal center while eliminating the remote π-electron delocalization has become a key challenge in the design of SACs.
 Highlights
Highlights
1. Innovative Synthesis Strategy:Selective cleavage of the C-C bonds of the peripheral phenyl rings of NiTPP through temperature gradient pyrolysis, preserving the first shell of Ni-N4 and the second shell of C, precisely eliminating long-range π-electron delocalization, achieving controllable electronic structure modulation.
2. Outstanding Catalytic Performance:The optimized catalyst maintains 98.3% CO selectivity at an industrial-level current density (500 mA cm-2) and achieves a 29-fold increase in FE (85.9% vs 2.97%) at -1.4 V vs RHE.
3. In-Depth Mechanistic Analysis:Combining in situ infrared spectroscopy with DFT calculations reveals that after the elimination of long-range π-electron delocalization, the electron density at Ni sites increases, lowering the adsorption energy of the *COOH intermediate, significantly accelerating the CO generation pathway.
 Graphical Analysis
Graphical Analysis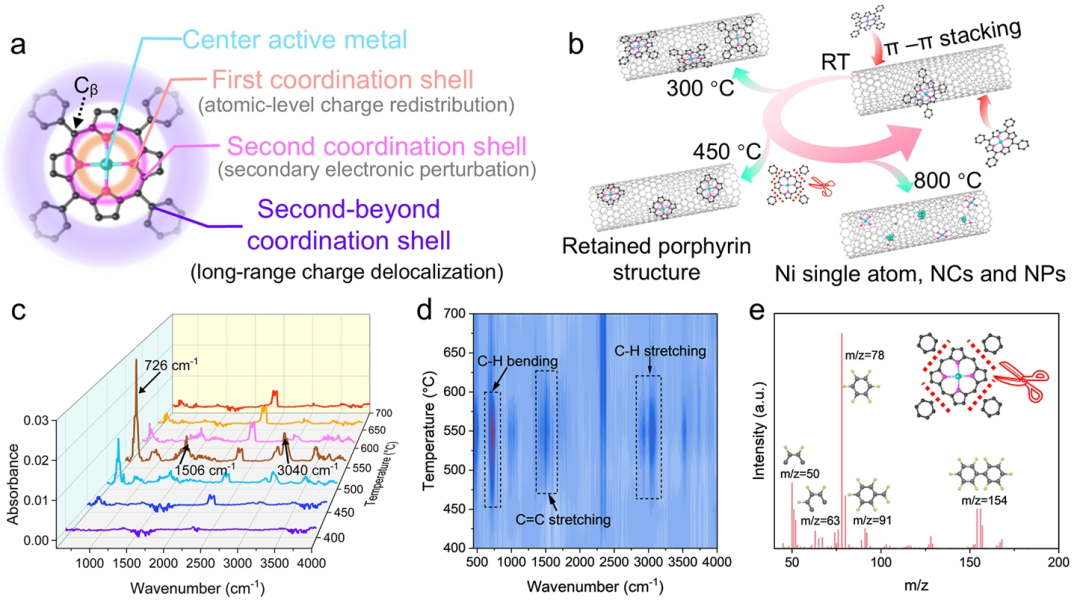
Figure 1. Mechanistic Analysis of NiTPP@CNT Synthesis.(a) Schematic diagram of the molecular structure of NiTPP, highlighting the central active metal (Ni), first coordination shell (N), second coordination shell (C), and higher coordination shells. Cyan spheres represent Ni atoms, pink spheres represent N atoms, and black spheres represent C atoms.(b) Schematic diagram of the molecular structure evolution of NiTPP loaded on the surface of carbon nanotubes during pyrolysis as temperature changes.(c) Infrared spectra of gaseous volatiles produced during the pyrolysis of NiTPP molecules in the temperature range of 400 to 700°C.(d) Corresponding contour plot of the infrared spectra.(e) Pyrolysis-gas chromatography-mass spectrometry (PY-GC-MS) analysis results of NiTPP@CNT prepared at 450°C.
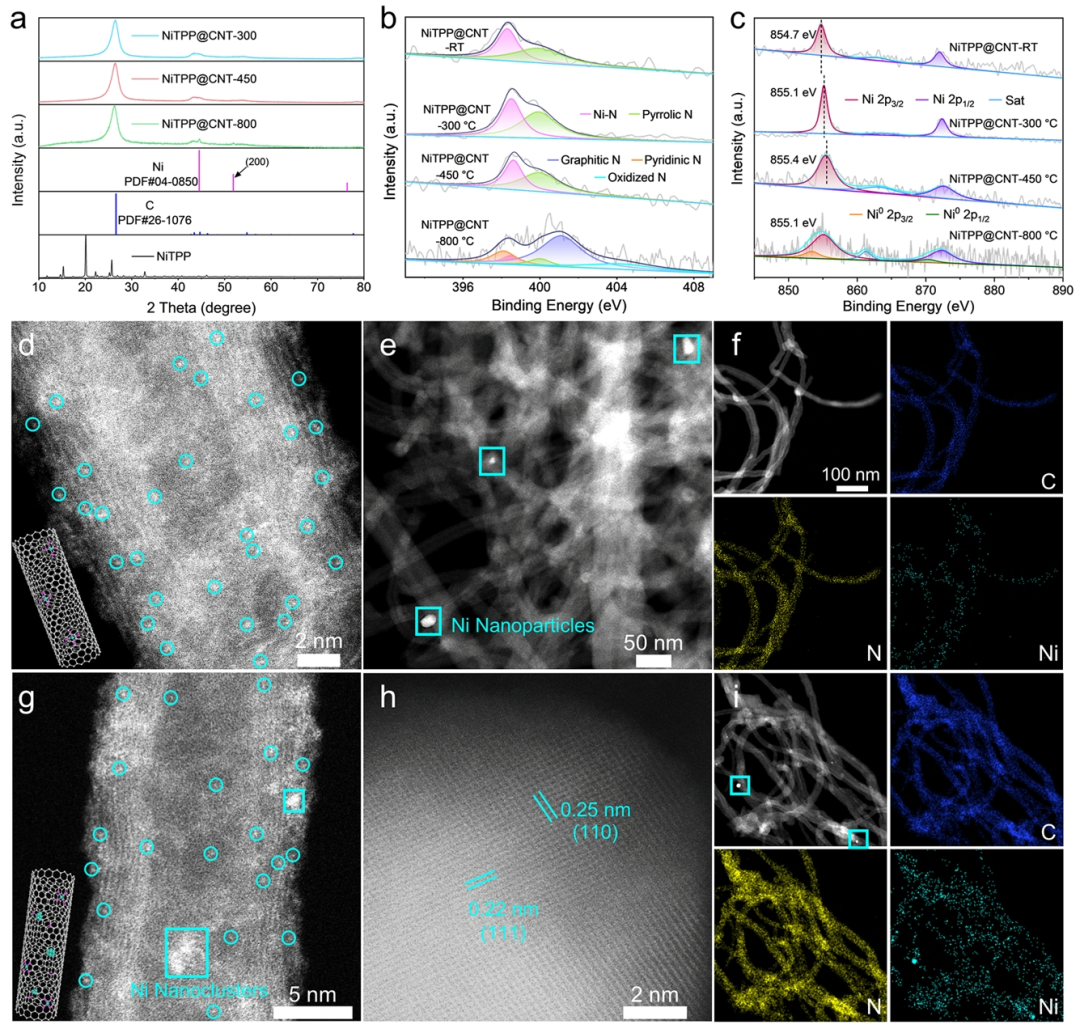
Figure 2. Microscopic Structural Characterization of NiTPP@CNT.(a) XRD patterns of NiTPP@CNT-300, 450, and 800.(b) N 1s XPS spectrum.(c) Ni 2p XPS spectrum.(d) HAADF image of NiTPP@CNT-450, with Ni single atoms highlighted in cyan circles.(e) HAADF image of NiTPP@CNT-800, with Ni nanoparticles highlighted in cyan squares.(f) HAADF-STEM image of NiTPP@CNT-450 and its corresponding EDS elemental distribution map (C, N, Ni).(g) HAADF image of NiTPP@CNT-800, with Ni nanoparticle clusters highlighted in cyan squares.(h) HAADF image of Ni nanoparticles.(i) HAADF-STEM image of NiTPP@CNT-800 and its corresponding EDS elemental distribution map (C, N, Ni).
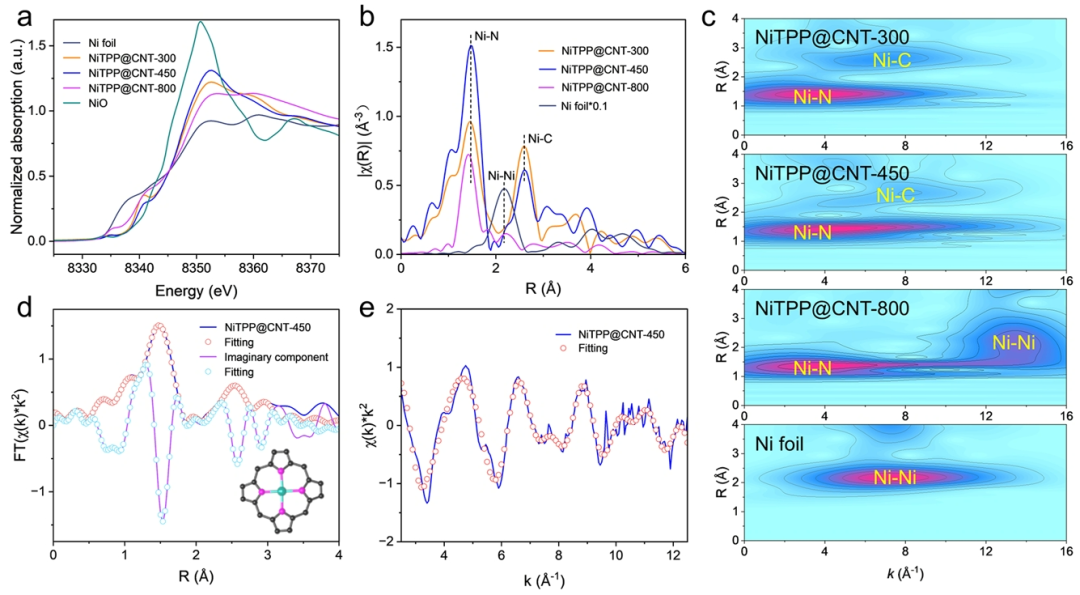
Figure 3. Structural Characterization of NiTPP@CNT.(a) Ni K-edge X-ray absorption near-edge structure (XANES) spectra of NiTPP@CNT-300, 450, and 800.(b) Ni K-edge extended X-ray absorption fine structure (EXAFS) spectra of NiTPP@CNT-300, 450, and 800.(c) Wavelet transform EXAFS spectra of NiTPP@CNT-300, 450, 800, and Ni foil.(d) Experimental data and fitting curves of Ni K-edge R-space for NiTPP@CNT-450.(e) Experimental data and fitting curves of Ni K-edge k-space for NiTPP@CNT-450.
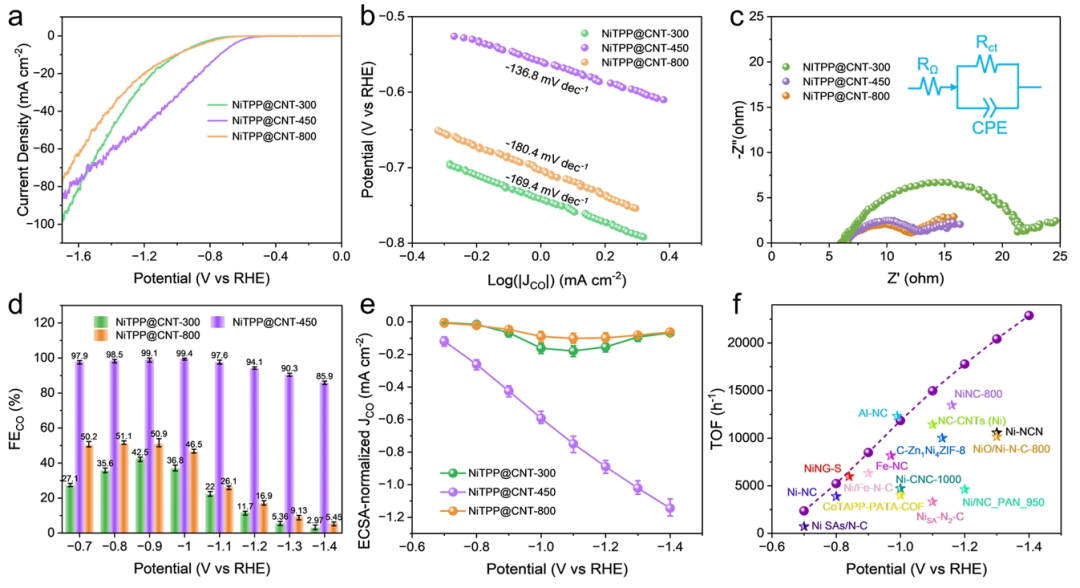
Figure 4. CO2 Reduction Performance of NiTPP@CNT in H-Type Electrolyzer.(a) Linear sweep voltammetry (LSV) curves of NiTPP@CNT-300, 450, and 800 in CO2 saturated electrolyte.(b) Comparison of Tafel slopes.(c) Nyquist impedance spectra, with the inset showing the corresponding equivalent circuit model.(d) Variation trend of CO Faradaic efficiency (FECO) with applied potential.(e) Normalized CO partial current density (JCO) based on electrochemical active surface area (ECSA).(f) Comparison of CO conversion frequency (TOF) of NiTPP@CNT-450 with existing catalysts.
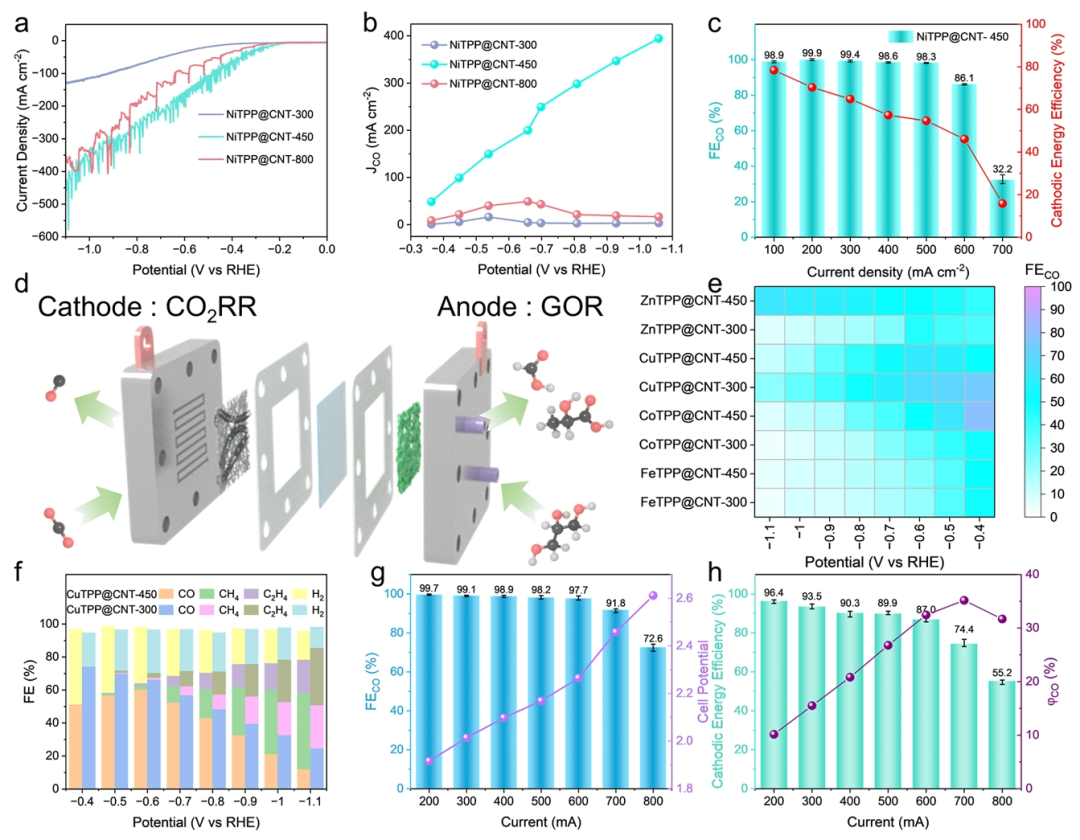
Figure 5. CO2 Reduction Performance of NiTPP@CNT-450 in Flow Cell and Membrane Electrode Assembly (MEA).(a) Linear sweep voltammetry (LSV) curves of NiTPP@CNT-300, 450, and 800 tested in flow cell.(b) Corresponding CO partial current density (JCO) and (c) CO Faradaic efficiency (FECO) compared to cathodic energy efficiency.(d) Schematic diagram of MEA device, with glycerol oxidation reaction (GOR) occurring at the anode.(e) Heatmap analysis of CO Faradaic efficiency (FECO) of different metal single-atom catalysts.(f) Comparison of CO2 reduction performance of CuTPP@CNT-450 and CuTPP@CNT-300.
(g) Relationship between FE(%) of NiTPP@CNT-450 at different currents and voltage.
(h) Variation trend of CO volume fraction (φCO) in the cathode output gas and cathodic energy efficiency with current.
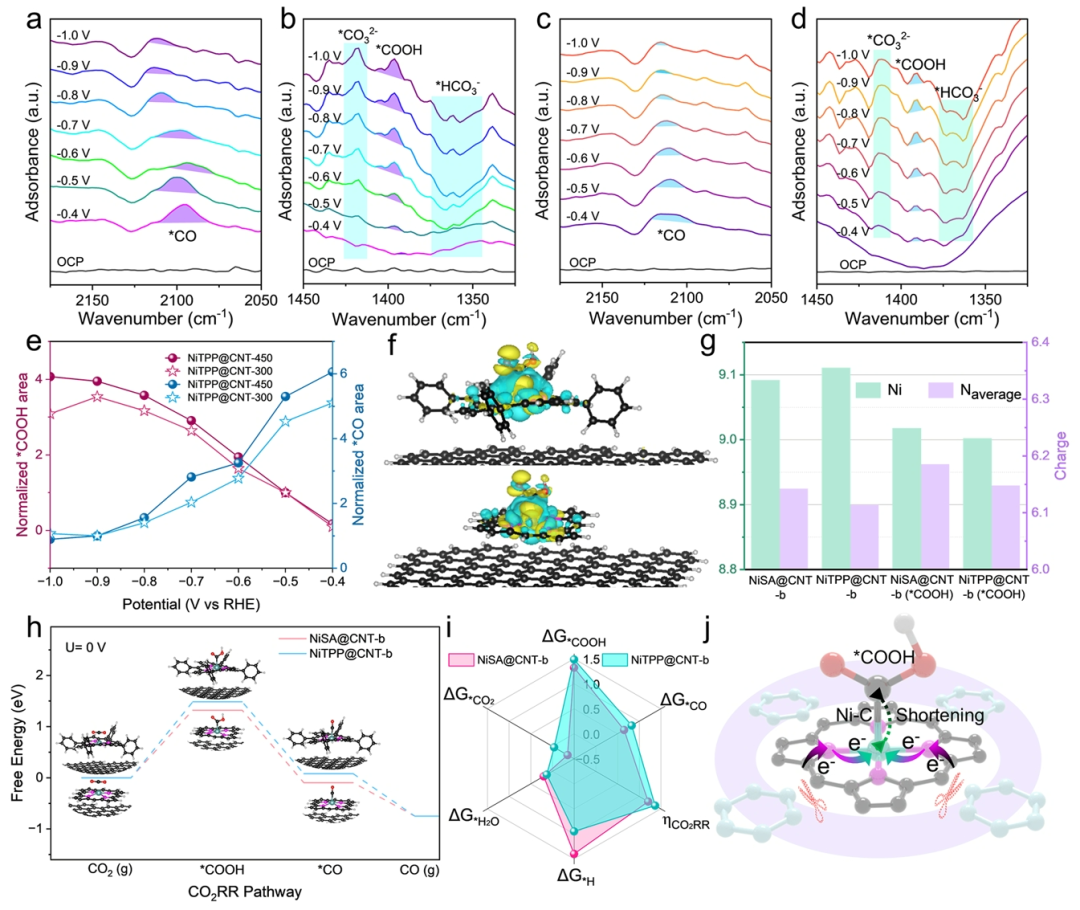
Figure 6. Mechanistic Study of CO2 Reduction Performance of NiTPP@CNT-450.(a) and (b) In situ ATR-SEIRAS spectra of NiTPP@CNT-450 in the potential range of -0.4 to -1.0 V.(c) and (d) In situ ATR-SEIRAS spectra of NiTPP@CNT-300 in the potential range of -0.4 to -1.0 V.(e) Normalized comparison of *COOH and *CO signal peak areas of NiTPP@CNT-450 and NiTPP@CNT-300.(f) Charge density difference analysis of NiTPP@CNT-b and NiSA@CNT-b (yellow areas indicate charge accumulation regions, cyan areas indicate charge depletion regions).(g) Average charge density of the central Ni and N atoms of the two catalysts before and after the adsorption of COOH species.(h) Free energy changes of CO generation pathways on the two catalysts.(i) Comparison of multiple performance indicators of the two catalysts.(j) Electron transfer trajectories during the adsorption process of COOH species after shielding long-range π-electron interactions.
 Conclusion and Outlook
Conclusion and Outlook
This study achieved controllable modulation of higher coordination shells in single-atom catalysts through precise pyrolysis strategies, revealing the critical impact of long-range π-electron delocalization on CO2 electroreduction performance. Future research directions include:
1. Industrial Adaptability:Developing scalable synthesis methods to promote the practical application of CO2 electrolysis at high current densities.
2. Multiscale Simulation:Combining machine learning with multiphysics simulations to optimize design principles for higher coordination shell engineering.
 References
References
Wang L, Fu S, Shi R, et al. Beyond Second Coordination Shell: Long-Range π-electrons Delocalization Engineering in Single-Atom Catalysts for CO2 Electroreduction. Angew. Chem. Int. Ed. 2025. DOI: 10.1002/anie.202506663
Article URL:https://doi.org/10.1002/anie.202506663
 Research Group Introduction
Research Group Introduction
Wu Yuen:Professor at the University of Science and Technology of China, PhD supervisor, and Changjiang Scholar of the Ministry of Education. He obtained his bachelor’s and doctoral degrees from Tsinghua University from 2005 to 2014 and joined USTC as a tenure-track associate professor in 2014. In recent years, he has focused on the rational design and fine-tuning of single-atom and cluster catalysts, applying them to the precise activation of small molecule “chemical bonds” in energy and catalysis. He received the Excellent Young Fund from the National Natural Science Foundation in 2015, led a youth project funded by the National Key R&D Program in 2017, received support as a top young talent from the Organization Department of the CPC Central Committee in 2017, won the Nano Chemistry Rising Star Award from the Chinese Chemical Society in 2018, the Young Chemist Award from the Chinese Chemical Society in 2019, the Ho Leung Ho Lee Foundation Young Teacher Award in 2020, and the Young Scientist Award from the Royal Society of Chemistry in 2025. Since 2015, he has published over 150 academic papers as corresponding author (including co-corresponding), with over 28,000 citations in the last five years, an h-index of 78, and was selected as a Highly Cited Researcher by Clarivate Analytics from 2020 to 2023. He serves as the deputy editor of the international journal Science Bulletin (Q1), editorial board member of Science China Materials (Q1), editorial board member of Industrial Chemistry & Materials, guest editor of Small Methods, and youth editorial board member of the Journal of Inorganic Chemistry, among other roles. He has achieved multiple results in technology transfer, and products derived from single-atom catalysts are now applied in companies such as Midea, Xiaomi, Tianbang, Zhuimi, and Panasonic.
Research Group Homepage:http://staff.ustc.edu.cn/~yuenwu/
Yu Zhenqiang:Professor and PhD supervisor at the School of Chemistry and Environmental Engineering, Shenzhen University. He has long been engaged in research related to functional liquid crystals (luminescent liquid crystals, chiral liquid crystals, polymer liquid crystals, circularly polarized luminescence, and nano-imprinting guided self-assembly/co-assembly), providing theoretical and methodological guidance for the design, synthesis, performance, and application of functional liquid crystals through structure-performance relationship studies. He has led or completed multiple projects funded by the National Natural Science Foundation, Guangdong Natural Science Foundation, and Shenzhen Science and Technology Plan, with related research published in journals such as Nat. Commun.、Angew. Chem. Int. Ed.、Adv. Mater.、Chem. Sci.、ACS Nano、ACS Mater. Lett.、Small、Science China-Materials and other domestic and international professional journals.

FollowEnvironmental Materials Insights provides live services for meetings, lectures, etc.!
WeChat Group:
Environmental Materials Insights provides a platform for information exchange and sharing for experts, scholars, and researchers in the field of environmental materials development. We have established a professional discussion group in the hot areas of environmental materials, and we welcome scholars and graduate students to join.

To join the group: Scan the QR code below to add the editor as a friend and get invited to the group. Please note: Name – Institution – Research Direction.
Scan the QR code to add the editor on WeChat, get invited to the group, and receive more information.
