
The polarity of free radical intermediates has a profound impact on their reactivity and selectivity. To quantify this effect and predict its outcomes, the Nagib research group at Ohio State University calculated the electrophilicity/nucleophilicity of over 500 types of free radicals. The figure also includes a simple “rule of thumb” for qualitatively predicting free radical polarity, where nucleophilic radicals are more easily oxidized to stable cations, while electrophilic radicals are more readily reduced to their anions.
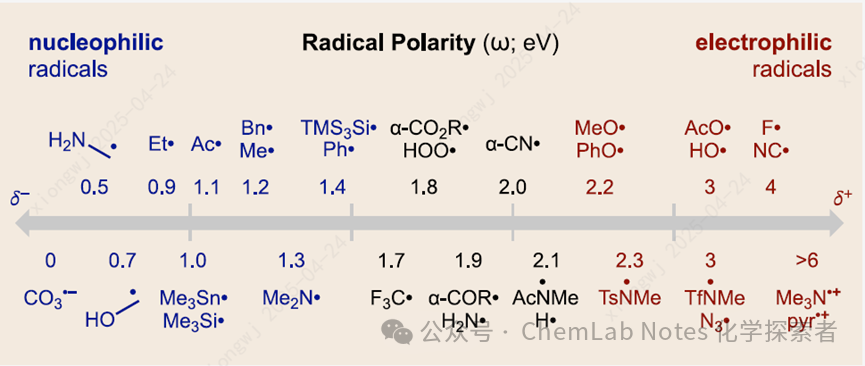
The research group compiled a list of the most common free radicals in organic synthesis and calculated their electrophilicity (ω) at the B3LYP-D3/6-311 + G** theoretical level. The fundamental principle for selecting these free radicals was to assess various key effects, including atomic hybridization (sp3, sp2, sp) and different spatial and electronic substitutions—across a wide range of structures typically found in synthetic and biologically relevant molecules. To illustrate the depth and breadth of these selected free radicals, two figures are included below, showing the electrophilicity of different categories of free radicals centered around carbon and various heteroatoms (O, N, S, P, B, Si, X) as the center. At the bottom, the electrophilicity scale (in eV) shows an increase in electrophilicity from left to right. To calibrate each chart, H• (approximately 2 eV) is displayed on the central vertical line. Different categories of free radicals are clustered and vertically separated for quick identification, with the simplest and most typical displayed at the top, gradually increasing in complexity downwards. Within each row, the substituent effects are graphically highlighted by including key structures, while each data point is represented by a dot on the line.
Figure 1. Free radical polarity centered around carbon; from nucleophilicity (left) to electrophilicity (right).
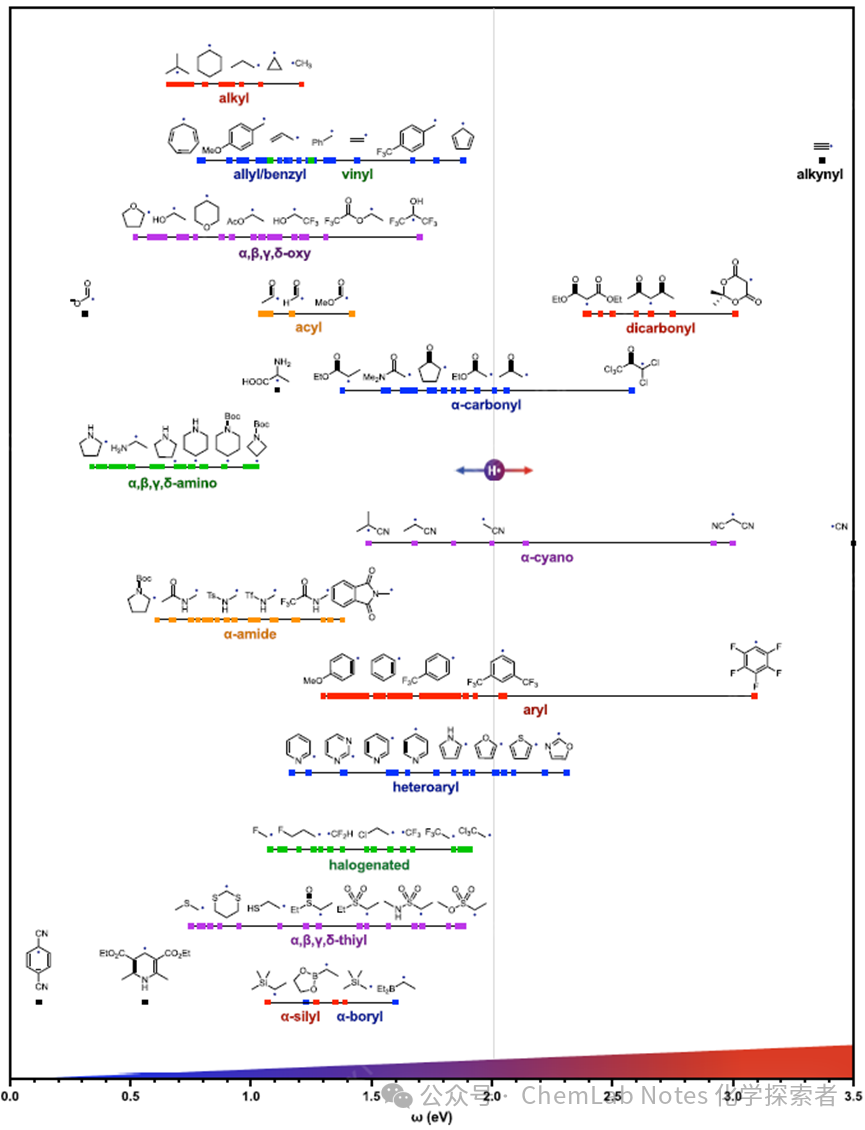
Figure 1 Detailed Description: First row: Red, alkyl radicals. The figure shows 16 alkyl radicals represented as 16 red dots (approximately 1 eV), with structures of five representative alkyl radicals shown along the line, illustrating that greater substitution on the C centered radical decreases electrophilicity (or increases nucleophilicity).
Second row: Blue, allylic and benzylic radicals, with key substituent effects shown in the para-substituted benzylic radicals, and five-membered and seven-membered rings that can form aromatic or anti-aromatic molecules after oxidation. For comparison, vinyl (green) and acetylenic (black) radicals are also included in this row, the latter highlighting the extremely high electrophilicity of sp hybridized radicals (>3 vs 1 eV; see nitrile).
Third row: (Purple) Carbon radicals substituted with heteroatoms, with oxygen substitutions located at different positions—adjacent to alcohols, ethers, or esters, such as α, β, γ, δ–oxygen. These are generally more nucleophilic than their aliphatic counterparts.
Fourth and fifth rows: Acyl (orange) radicals show significant nucleophilicity, while α–carbonyl (blue) and especially β–dicarbonyl (red) show electrophilicity.
Seventh, eighth, and ninth rows: A wide range of nitrogen-substituted radicals, including nucleophilic α, β, γ, δ–amino (green) and α–amide (orange) C radicals, compared to electrophilic α–cyano (purple) radicals.
Finally, other heteroatom substituents are shown for comparison, including halogens (green), sulfur in different oxidation states (thio; purple), as well as α–silicon (red) and α–boron (blue) carbon radicals. Importantly, common charged and neutral radicals, due to their nucleophilicity, are often used as single-electron reductants, also included on the left side for reference (e.g., formate (top), and radicals from Hantzsch esters or dicyanobenzene (bottom)).
Atoms with electronegativity greater than carbon (N, O) lead to higher electrophilicity, as shown in the broader scale in Figure 2 (20 eV vs 3 eV). Conversely, atoms with lower electronegativity (B, Si) produce more nucleophilic radicals (<1.5 eV).
Figure 2. Polarity of heteroatom-centered free radicals; from nucleophilicity (left) to electrophilicity (right).
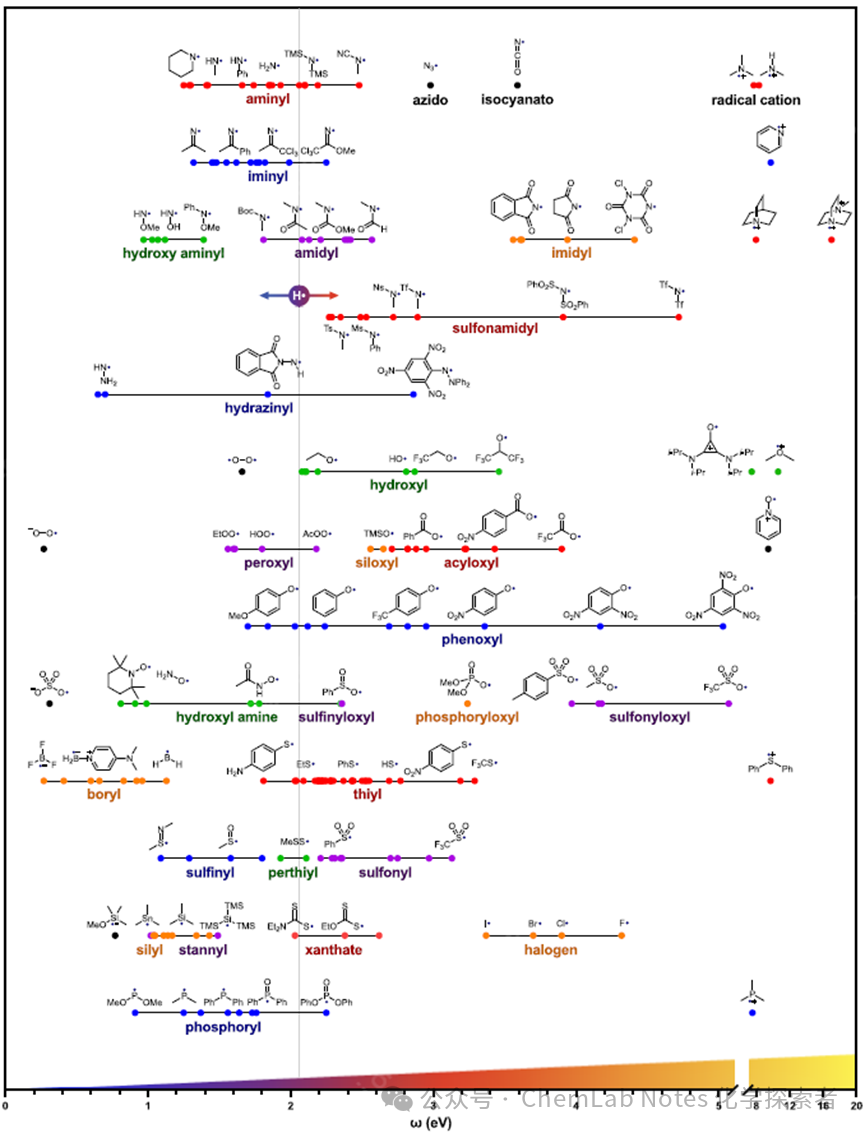
In Figure 2, the first five rows contain ten different categories of nitrogen-centered radicals (amino, azido, isocyanide, cationic amino radicals, imino, hydroxylamino, amido, imidazole, sulfonamide, hydrazine) and their respective clusters (1−5 eV), demonstrating the greater importance of atomic, hybridization, or functional group identity on free radical polarity than their substituents. When nitrogen-centered radicals are substituted with alkyl groups, they are primarily nucleophilic (<2 eV, such as amino (red) or imino radicals (blue). α–heteroatoms (N, O) also impart nucleophilicity, such as hydroxylamino (green) and hydrazine radicals (blue). Otherwise, any substituent that increases the p character of the nitrogen-centered radical will yield the expected electrophilicity (>2 eV). For example, azido and isocyanide radicals (black), as well as nitrogen radicals substituted with carbonyl or sulfonyl groups are almost all to the right of H• (more electrophilic). The third row of this chart illustrates a key point that can be quickly gleaned from this graphical representation; all imidazole radicals (orange; 4 eV) are more electrophilic than amido or carbamate radicals (purple; 2 eV), regardless of substituents. However, sulfonamide substitutions (red; 2−5 eV) have a significant impact on ω, producing radicals that are either more electrophilic than all amido radicals (Tf2N•; 5 eV), or less electrophilic than some amido and carbamate radicals (TsMeN•; 2.3 eV).
In the upper right, several common nitrogen radical cations (red) are included, which are typically used as single-electron oxidants or HAT media (8−17 eV; note the broken axis, including to show scale), to demonstrate the significant influence of formal charge. Notably, each of these radical cations is more electrophilic than the most electronegative element, fluorine (>8 vs 4 eV).
The sixth to ninth rows of the above figure display 14 different categories of oxygen-centered radicals, where the oxygen atom is covalently bonded to C, O, N, P, S, Si, acyl, aryl, or sulfonyl groups. As expected, almost all of these oxygen-centered radicals are more electrophilic than hydrogen (>2 eV), with TfO• (5 eV) and 2,4,6- (NO2)3-PhO• (5 eV) are more electrophilic than fluorine (4 eV).
Interestingly, some outliers exhibit α–heteroatom substitution effects, where heteroatoms bonded to oxygen are electron-releasing, such as oxygen (neutral or anionic; black), peroxyl (purple), and hydroxylamine (green), all of which are less electrophilic than hydrogen (<2 eV). In contrast, carbon, silicon, or sulfur substitutions merely modulate (or enhance) the expected electrophilicity, such as hydroxyl (green), siloxy (orange), acyloxy (red), phenoxy (blue), sulfinyl (purple), and phosphonyl (orange) radicals. Similarly, charged examples, such as oxygen radical cations (green or black), are the most electrophilic, exceeding the broken axis range (>8 eV).
Finally, the last four rows showcase other common heteroatom radicals frequently used in synthesis, including radicals centered around boron (boronyl; orange), sulfur (thio; red, including different oxidation states such as sulfinyl (blue), persulfide (green), sulfonyl (purple), and thiol (red)), silicon (silyl; orange), tin (stannyl; purple), and phosphorus (phosphonyl; orange) radicals. Interestingly, all of these heteroatom-centered radicals (S, Si, Sn, P) are less electrophilic than halogen radicals (>3 eV) and more nucleophilic (<3 eV). Similarly, radical cations (such as radical cations on sulfur or phosphorus, similar to those on nitrogen and oxygen above) are highly electrophilic outliers (>8 eV), exhibiting oxidizing properties, while radical anions (such as radical anions on oxygen, boron, or silicon) are highly nucleophilic (<0.8 eV), which can be used as single-electron reductants.
The article is quite lengthy, and we will discuss different types of free radicals in detail over the next few days. Interested readers can like, follow, and bookmark.
References:
https://doi.org/10.1021/jacs.4c06774
J. Am. Chem. Soc. 2024, 146, 28034−28059
Note: All images in this article are sourced from the reference. If there are any copyright issues, please contact the author promptly for proper handling. If you have any questions about this literature, feel free to communicate and discuss with the editor.