
Continuing from the previous article, this section will further introduce the types of radicals.The research team from Ohio State UniversityNagibhas summarized the polarity of carbon-centered radicals and oxygen-substituted carbon-centered radicals.
Carbon-centered radicals:The table below1lists the polarities of various aliphatic radicals.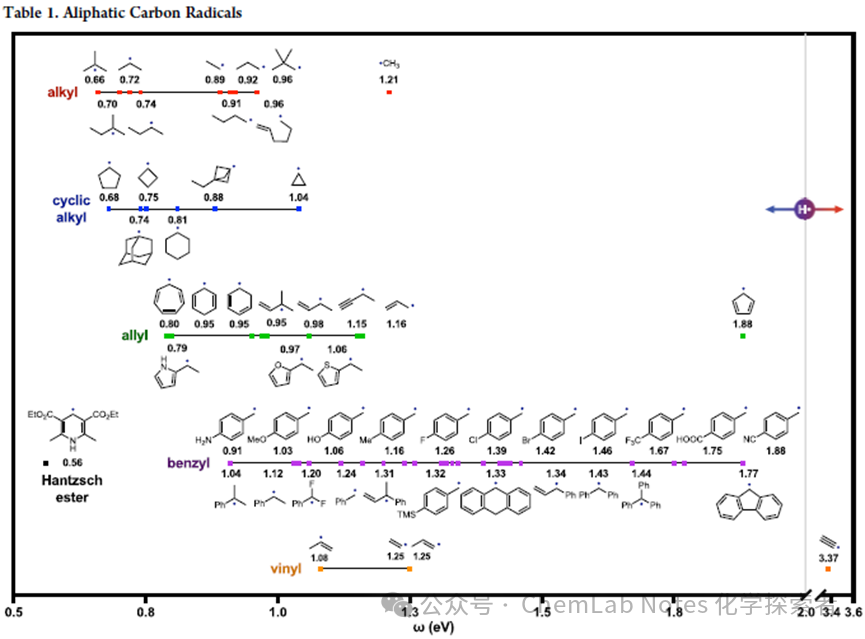 All of these alkyl radicals are more nucleophilic than hydrogen (<2 eV), but key trends can still be derived from the table. For example, simple alkyl (sp3) radicals are very nucleophilic regardless of substitution pattern or cyclic/non-cyclic arrangement (red/blue), with nucleophilicity (<1 eV). Then, to varying degrees, methyl (red), allyl (green), benzyl (purple), and vinyl (orange) radicals have lower nucleophilicity but are still more nucleophilic than hydrogen (<2 eV). Significant observations from this data include:
All of these alkyl radicals are more nucleophilic than hydrogen (<2 eV), but key trends can still be derived from the table. For example, simple alkyl (sp3) radicals are very nucleophilic regardless of substitution pattern or cyclic/non-cyclic arrangement (red/blue), with nucleophilicity (<1 eV). Then, to varying degrees, methyl (red), allyl (green), benzyl (purple), and vinyl (orange) radicals have lower nucleophilicity but are still more nucleophilic than hydrogen (<2 eV). Significant observations from this data include:
1.Substituent Effects: The nucleophilicity increases from methyl radical (1.2 eV) to primary (0.9 eV) and secondary/tertiary radicals (0.7 eV), illustrating the influence of hyperconjugation/inductive effects on increasing the electron density of the radical carbon. Similar substituent effects are also present in the benzyl series (nucleophilicity: 3° > 2° > 1°; 1.0, 1.1, 1.2 eV), as well as in the allyl radical series: 3°/2° > 1°; 1.0, 1.2 eV.
2.Strain Effects: In the family of cyclic alkyl radicals, nucleophilicity decreases with increasing ring strain. For example, five-membered rings (0.7 eV), four-membered rings (0.8 eV), or three-membered rings (1 eV) become less nucleophilic in that order. Outliers, such as cyclohexyl (0.8 eV), adamantyl (0.7 eV), and bicyclopentyl (0.9 eV) are less nucleophilic than expected—possibly due to overlap with adjacent antibonding orbitals (e.g., σC−H or σC−C).
3.Resonance Effects: Electron delocalization also decreases nucleophilicity. Guiding comparisons include:(i) propyl (0.9 eV) versus allyl (1.2 eV), (ii) cyclohexyl (0.8 eV) versus cyclohexadienyl (1 eV), and (iii) benzyl (1.1 eV) versus dibenzyl/tribenzyl (1.4 eV).
4.Aromaticity: A useful mnemonic for understanding polarity includes observing that nucleophilic radicals can be easily oxidized to stable cations, while electrophilic radicals can be easily reduced to stable anions. An example includes cycloheptadienyl (0.8 eV), a nucleophilic radical that oxidizes to an aromatic (stable) cation, compared to cyclopentadienyl (1.9 eV), an electrophilic radical that reduces to an aromatic anion. Notably, easily oxidized and nucleophilic pre-aromatic radicals (e.g., cyclohexadienyl, 1.0 eV; Hantzsch ester, 0.6 eV) are often used in synthesis.
5.Benzyl Substituents: To correlate this database with other resources, the Nagib research team calculated the electrophilicity of benzyl radicals with different para-substituents, whose Hammett constants (σp) have been rigorously experimentally measured (albeit for bi-electronic systems) and are widely available. Encouragingly, the plot of calculated electrophilicity (ω) versus experimentally measured σp shows a strong correlation (R²= 0.94) (see above figure1). Notably, benzyl radicals with para donor groups:NH2 (0.9 eV), OMe (1.0 eV), Me (1.16 eV) are more nucleophilic than unsubstituted phenyl, H (1.2 eV) with negative σp values (<0). Conversely, benzyl radicals with para acceptor groups:F (1.3 eV), CF3 (1.7 eV), CN (1.9 eV) are more electrophilic, correlating with their positive σp values (>0).
6.Hybridization:: The s-character of the radical’s SOMO increases with higher electrophilicity. For example, an sp3 radical (ethyl: 0.9 eV) is more nucleophilic than an sp2 vinyl radical (internal vinyl, 1.1 eV; terminal vinyl, 1.3 eV) which is more nucleophilic than an sp radical (acetylene: 3.4 eV).
7.Effects are Cumulative: Notably, these effects are retained and can be combined, for example, in the case of tertiary/secondary/primary alkyl (3° > 2° > 1°) being more nucleophilic than similar allyl or benzyl triplets, which follow the same trend within their respective groups.
Oxygen-substituted carbon-centered radicals: The table below2 lists the polarities of oxygen-substituted carbon-centered radicals.
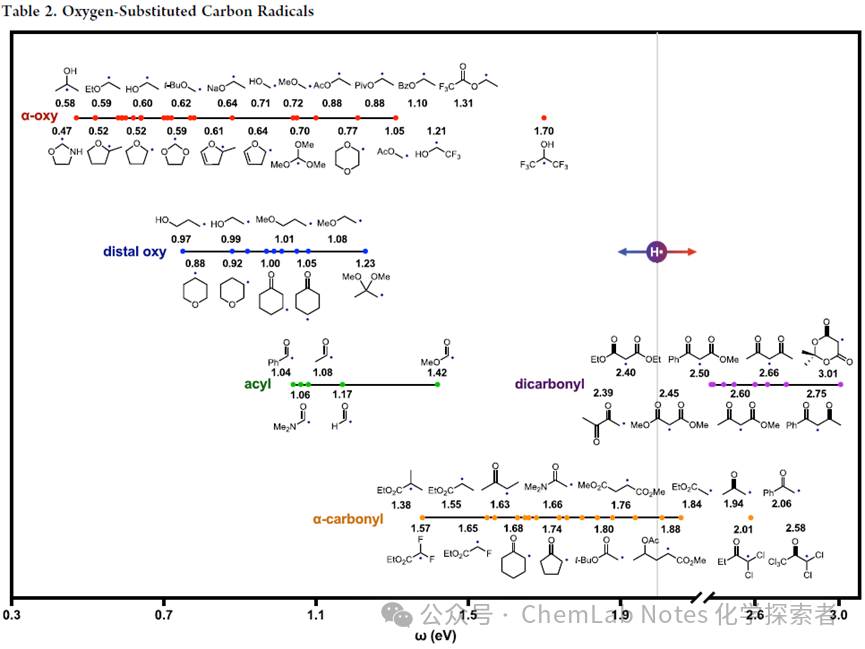
Compared to non-heteroatom-substituted alkyl radicals, these oxygen analogs are generally more nucleophilic, with α– and multi-substituted (red) effects being more significant, while distal (β, γ) or mono-substituted (blue) effects are less pronounced. As shown by Roberts’ pioneering contributions, acyl radicals (green) are more nucleophilic than α– carbonyl analogs (orange). The higher electrophilicity of dicarbonyl radicals (purple) again indicates that these effects are cumulative. Notable observations also include:
1.Resonance Effects: When α– oxygen substitution allows the oxygen lone pair electrons to resonate and donate electrons to the radical, nucleophilicity is enhanced. For example, tetrahydrofuran (THF; 0.5 eV) is more nucleophilic than cyclopropyl (0.7 eV), and α– diethyl ether (0.6 eV) is more nucleophilic than secondary butyl (0.7 eV). In contrast, substituents on THF have less impact on tertiary and secondary (both 0.5 eV) but more significant effects on secondary versus primary (0.6 vs 0.7 eV). Notably, α– acetals are less nucleophilic than simple α– ethers (0.6 vs 0.5 eV), possibly due to inductive effects complicating the polarity effects.
2.Inductive Effects: Conversely, distal oxygen atoms decrease nucleophilicity as their inductive effects are not balanced by resonance. For example, in cyclic and acyclic pairs (0.9−1.0 eV), β– oxygen radicals are slightly less nucleophilic than γ– oxygen radicals (<0.1 eV), and both are less nucleophilic than α– ethers (0.6 eV), which are more nucleophilic (>0.3 eV). Interestingly, acetals containing two β– oxygen groups are the most electrophilic in this group (1.2 eV).
3.Carbonyl Radicals: Given the ongoing interest of the Nagib research team in utilizing carbonyl radicals, Nagib has found that the nucleophilicity of carbonyl radical anions (0.64 eV) is slightly diminished compared to their protonated or alkyl counterparts (0.59−0.60 eV), although both are still more nucleophilic than simple alkyl radicals (0.72 eV). However, Nagib expects that the related acids or cation pairs have a significant impact on polarity—as observed in catalytic generation.
4.Dicarbonyl: Due to inductive effects, the α– radical of 1,4-diesters is more electrophilic than its monoester analogs (1.8 vs 1.6 eV). However, stronger resonance effects manifest in the significantly higher electrophilicity of 1,3-diesters (2.5 eV), where the α– radical is sandwiched between two carbonyls. Similarly, tracking acidity, the electrophilicity increases in this (fully methylated) series: β– diesters < β– diketones < β– ketoesters (2.5, 2.6, 2.7 eV).
The article is quite lengthy, and we will continue with detailed discussions on different types of radicals over the next few days. Interested readers can like, follow, and bookmark.
References:
https://doi.org/10.1021/jacs.4c06774
J. Am. Chem. Soc. 2024, 146, 28034−28059
Note: All images in this article are sourced from the references. If there are any copyright issues, please contact the author promptly for proper handling. If you have any questions about this literature, feel free to communicate and discuss with the editor.