Introduction
Recently, Professor Luis Echegoyen and others from the University of Texas at El Paso reported two covalent organic frameworks (COFs) based on Co(II)-porphyrin/[2,1,3]-benzothiadiazole (BTD), namely Co@rhm-PorBTD and Co@sql-PorBTD, and used them for O2 electrocatalytic reduction (ORR). The results show that both COFs exhibit 5.8 times (Co@rhm-PorBTD) and 1.3 times (Co@sql-PorBTD) higher mass activity compared to commercial Pt/C (20%), with activity being 10 times and 2.5 times that of Pt/C. The related work was published in ACS Nano titled “[2,1,3]-Benzothiadiazole-Spaced Co-Porphyrin-Based Covalent Organic Frameworks for O2 Reduction.”
Main Text
In the electrochemical conversion (EC) process, the oxygen reduction reaction (ORR) is crucial for energy storage and conversion. However, this reaction often requires the use of expensive platinum group metal (PGM) catalysts, which severely restricts the commercialization of fuel cell vehicles. Currently, chemists have adopted several strategies to reduce catalyst costs, including metal-free carbon materials (MFCMs), single-atom electrocatalysts (SAECs), and metal alloys. Among these, single-atom electrocatalysts have significant advantages due to their extremely high atomic utilization, catalytic activity, higher selectivity, and ease of integration into electrodes. To design ideal SAECs, two factors need to be addressed: (1) controlling appropriate coordination sites; (2) suitable material frameworks that can participate in effective electronic coupling of active sites.
Reticular chemistry provides opportunities to precisely adjust the spacing between atomic sites using various ligands, which has a significant impact on electrodynamics. Covalent organic frameworks (COFs) have high porosity and well-defined chemical structures, making them widely applicable in gas separation/storage, catalysis, and energy storage. The conjugated structure of COFs can enhance charge transport within the crystal, and suitable monomers can be selected as spacers to control the distance between atomic sites. Since 2015, redox-active porphyrin-based COFs have shone brightly as carriers for SAECs in various catalytic processes, including CO2 reduction reaction (CO2RR), hydrogen evolution reaction, oxygen evolution reaction, etc.
Here, Professor Luis Echegoyen and others from the University of Texas at El Paso reported two covalent organic frameworks (COFs) based on Co(II)-porphyrin/[2,1,3]-benzothiadiazole (BTD), namely Co@rhm-PorBTD and Co@sql-PorBTD, and used them for O2 electrocatalytic reduction (ORR). These cobalt-based SAECs exhibited excellent electrocatalytic efficiency for ORR. Due to the high electron affinity, BTD serves as a spacer group between the metal porphyrin moieties, effectively facilitating intermolecular charge transfer.
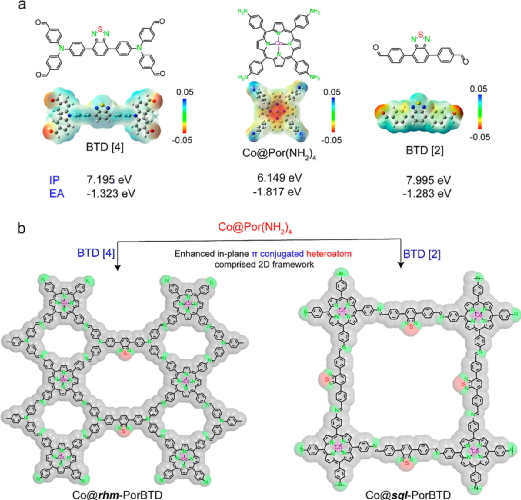
Figure 1. (a) Electrostatic potential (ESP) maps of various monomers; (b) Synthesis scheme of Co@rhm-PorBTD (left) and Co@sql-PorBTD (right).(Image source: ACS Nano)
As shown in Figure 1, by optimizing the amounts of catalyst (acetic acid) and the types of mixed solvents, two covalent organic frameworks (COFs) based on Co(II)-porphyrin/[2,1,3]-benzothiadiazole (BTD); Co@rhm-PorBTD and Co@sql-PorBTD were successfully synthesized, and the chemical structures of the materials were characterized by Fourier-transform infrared spectroscopy (FT-IR) and solid-state nuclear magnetic carbon spectroscopy (13CP-MAS).
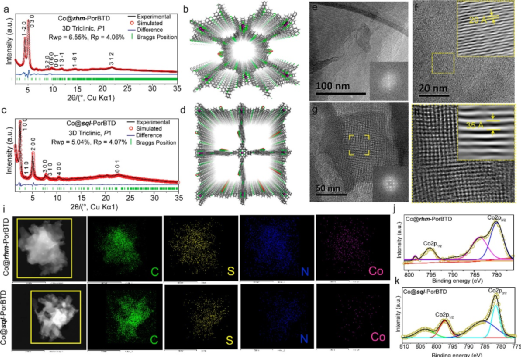
Figure 2. (a, b) PXRD patterns and simulated structure diagrams of Co@rhm-PorBTD; (c, d) PXRD patterns and simulated structure diagrams of Co@sql-PorBTD; (e-f) HR-TEM images of Co@rhm-PorBTD; (g-h) HR-TEM images of Co@sql-PorBTD; (i) Element mapping images of Co@rhm-PorBTD and Co@sql-PorBTD; (j) Co 2p XPS spectra of Co@rhm-PorBTD and (k) Co 2p XPS spectra of Co@sql-PorBTD.(Image source: ACS Nano)
Powder X-ray diffraction (PXRD) patterns indicate that Co@rhm-PorBTD and Co@sql-PorBTD exhibit good crystallinity. High-resolution transmission electron microscopy (HR-TEM) images show the formation of ordered structures in both COFs. Energy-dispersive X-ray spectroscopy (EDS) mapping analysis (Figure 2i) shows a uniform distribution of carbon (C), nitrogen (N), sulfur (S), and cobalt (Co). X-ray photoelectron spectroscopy (XPS) results indicate that the coordinated metal centers of the COFs are in the +2 state (Figure 2j, k).
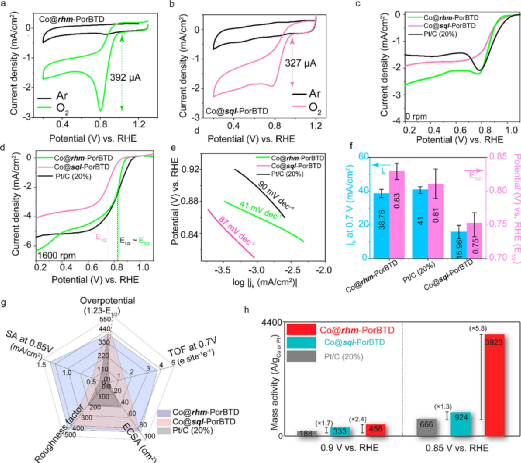
Figure 3. (a) CV curves of Co@rhm-PorBTD and (b) Co@sql-PorBTD in saturated argon (Ar) and O2 conditions in 0.1 M KOH solution; (c) ORR polarization curves at an electrode rotation speed of 0 rpm and scan rate of 10 mV s–1; (d) ORR polarization curves at an electrode rotation speed of 1600 rpm and scan rate of 10 mV s–1; (e) Tafel plots of Co@rhm-PorBTD, Co@sql-PorBTD, and Pt/C (20%); (f) Comparison of the current density and half-wave potential of Co@rhm-PorBTD, Co@sql-PorBTD, and Pt/C (20%); (g) Comparison of ECSA, half-wave potential overpotential (1.23- E1/2), and activity (0.85 V vs RHE), roughness; (h) Comparison of mass activities at different potentials.(Image source: ACS Nano)
Under O2 conditions, the reduction peaks of Co@rhm-PorBTD and Co@sql-PorBTD were observed (Figure 3a, b). The peak position of Co@rhm-PorBTD shows a slight positive shift (32 mV) compared to Co@sql-PorBTD. The reduction current of 20% Pt/C was 140μA, while the reduction currents of Co@rhm-PorBTD and Co@sql-PorBTD were 392 and 327μA, respectively, nearly double that of the commercial electrode. LSV curves of both COFs were recorded under static and dynamic conditions (Figure 3c, d). The onset potentials of Co@rhm-PorBTD and Co@sql-PorBTD were approximately 0.89 and 0.85 V (vs RHE), with half-wave potentials of 0.83 and 0.75 V (vs RHE), while the values for Pt/C (20%) were slightly higher than those of the COFs. The Tafel slopes of Co@rhm-PorBTD and Co@sql-PorBTD were 41 and 87 mV/dec, respectively (Figure 3e), significantly lower than that of 20% Pt/C (90 mv/dec), indicating that the COF-based SAC exhibited faster ORR kinetics than Pt/C (20%). The current density of Co@rhm-PorBTD was 38.7 mA/cm2, slightly lower than that of Pt/C (20%) (41 mA/cm2), while Co@sql-PorBTD showed a current density of 15.9 mA/cm2 (Figure 3f). A comprehensive comparison of the two synthesized COFs with commercial Pt/C (20%) was conducted (Figure 3g). At 0.9 V, the mass activities of Co@rhm-PorBTD and Co@sql-PorBTD were 458 A/g and 333 A/g, respectively, nearly 4.20 times and 1.7 times that of commercial Pt/C (20%) (Figure 3h), while at 0.85 V, the mass activities were 5.8 times and 1.3 times that of Pt/C. The SA values of Co@rhm-PorBTD (1.32 mA cm –2) and Co@sql-PorBTD (0.324 mA cm–2) were 10 times and 2.5 times higher than that of Pt/C (0.142 mA cm–2). This activity value exceeds the target value set by the U.S. Department of Energy for 2020.
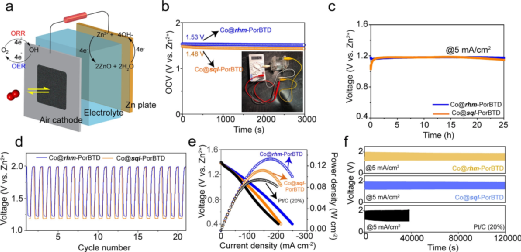
Figure 4. (a) Schematic diagram of a homemade zinc-air battery; (b) Open-circuit diagram of zinc-air batteries using Co@rhm-PorBTD and Co@sql-PorBTD as catalysts; (c) Discharge curves of zinc-air batteries using Co@rhm-PorBTD and Co@sql-PorBTD at a current density of 5 mA cm-2; (d) Discharge and charge cycle curves; (e) Comparison of polarization and power density curves using Pt/C (20%), Co@rhm-PorBTD, and Co@sql-PorBTD as cathodes; (f) Long-term discharge/charge cycle performance of zinc-air batteries containing Co@rhm-PorBTD, Co@sql-PorBTD, and Pt/C (20%) at a current density of 5 mA cm-2.(Image source: ACS Nano)
COF was coated on carbon cloth, and a zinc plate was used as an anode to assemble the battery (Figure 4a). The cathodes based on Co@rhm-PorBTD and Co@sql-PorBTD exhibited stable open-circuit voltages of 1.53 V and 1.48 V, respectively (Figure 4b). When discharged at a constant current of 5 mA/cm2, no significant voltage drop was observed for both COFs over 25 hours (Figure 4c). The charge and discharge voltages of Co@rhm-PorBTD were 1.99 and 1.22 V, while those of Co@sql-PorBTD were 1.99 and 1.18 V, respectively (Figure 4d). The peak power densities of Co@rhm-PorBTD and Co@sql-PorBTD were 138 and 117 mW cm–2, respectively, exceeding that of commercial Pt/C (20%) (96 mW cm–2) (Figure 4e). Long-term constant current charging/discharging showed no significant performance loss over 1000 hours of cycling, while the performance of Pt/C (20%) significantly degraded after 100 cycles (Figure 4f), indicating that the COF-based air cathodes exhibit superior stability compared to commercial Pt/C (20%).
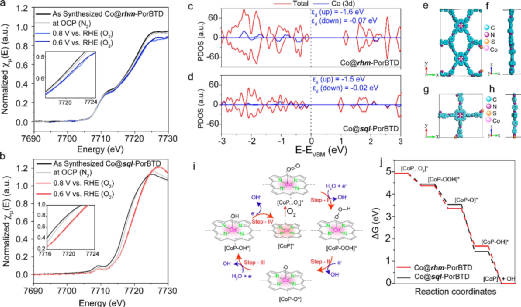
Figure 5. (a) Non-in-situ and in-situ XANES characterization of Co@rhm-PorBTD and (b) Co@sql-PorBTD; (c) Total density of states and projected density of states in Co@rhm-PorBTD and (d) Co@sql-PorBTD; (e) Top view and (f) side view of optimized Co@rhm-PorBTD monolayer; (g) Top view and (h) side view of optimized Co@sql-PorBTD monolayer; (i) Free energy diagrams of the two COFs; (j) Proposed ORR catalytic scheme.(Image source: ACS Nano)
Conclusion
Professor Luis Echegoyen and others from the University of Texas at El Paso developed two single-atom catalysts based on covalent organic frameworks, Co@rhm-PorBTD and Co@sql-PorBTD, using appropriate building blocks and suitable metal sites, which exhibit efficient ORR activity superior to many precious metal catalysts.
Literature Details:

Long press or scan the QR code on the left to view the original text