iNature
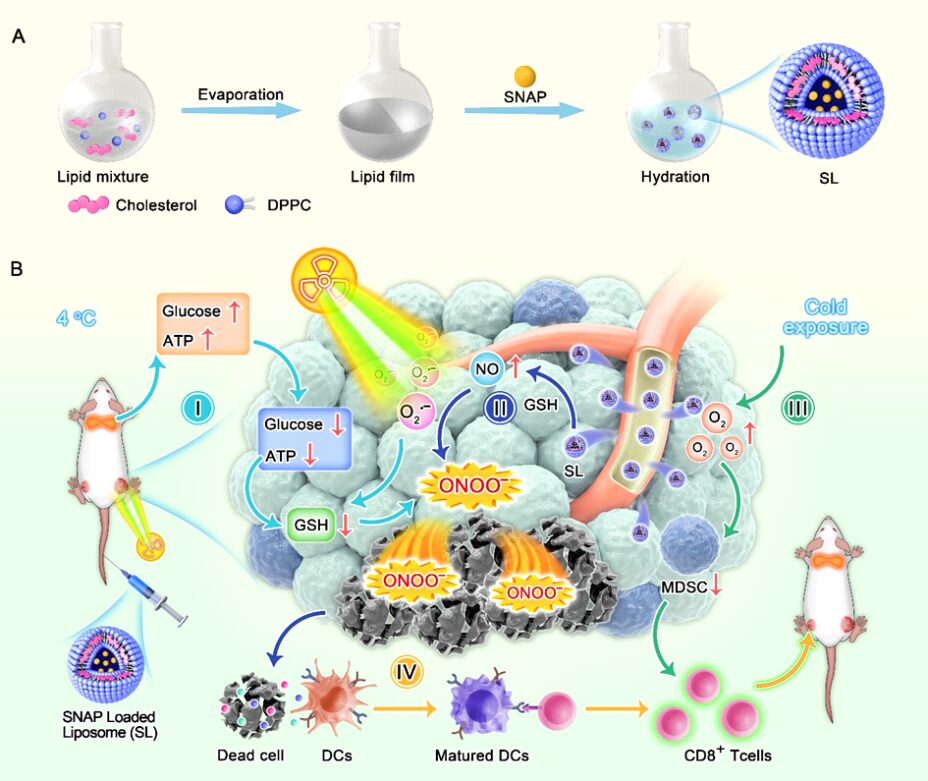
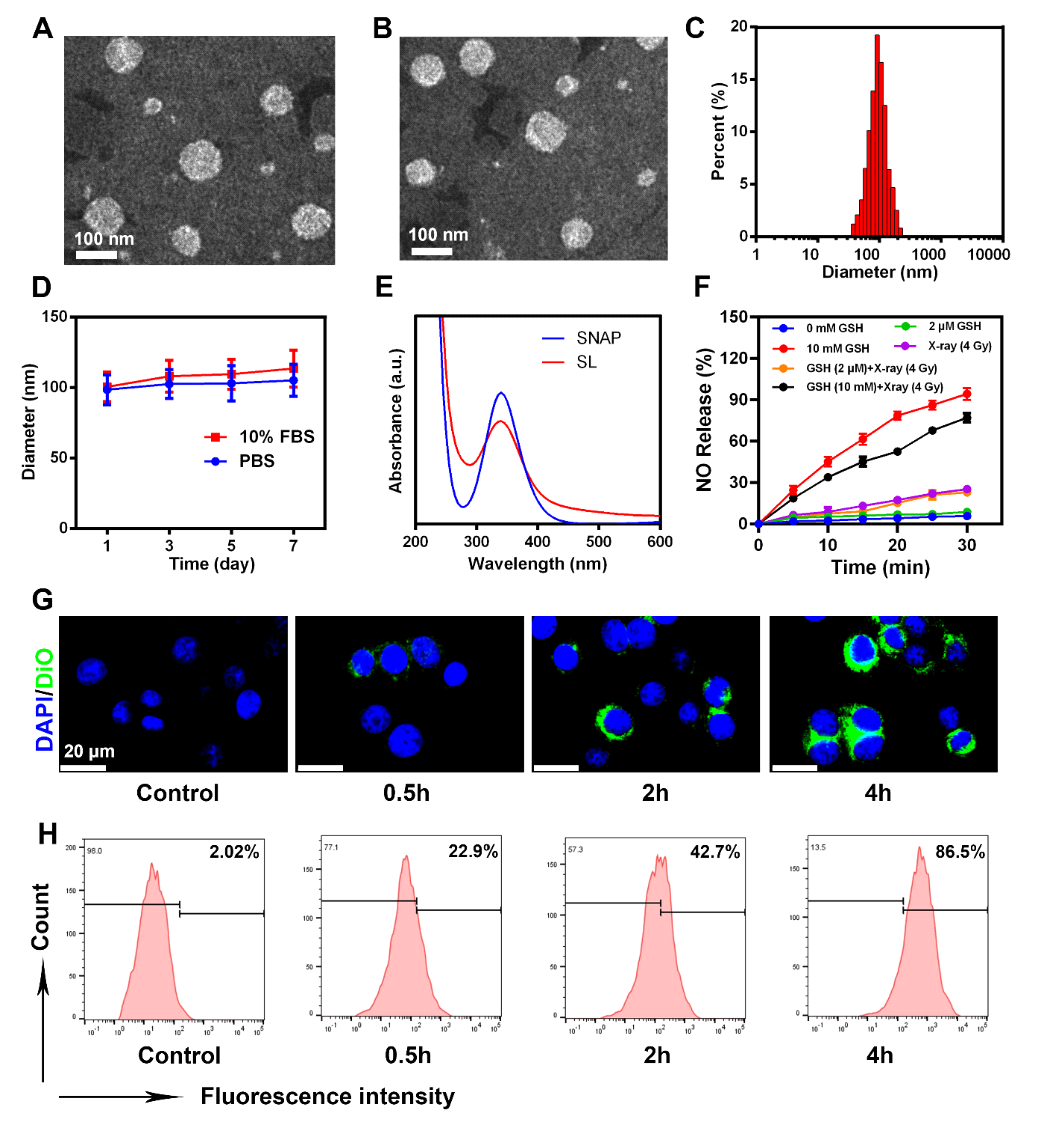
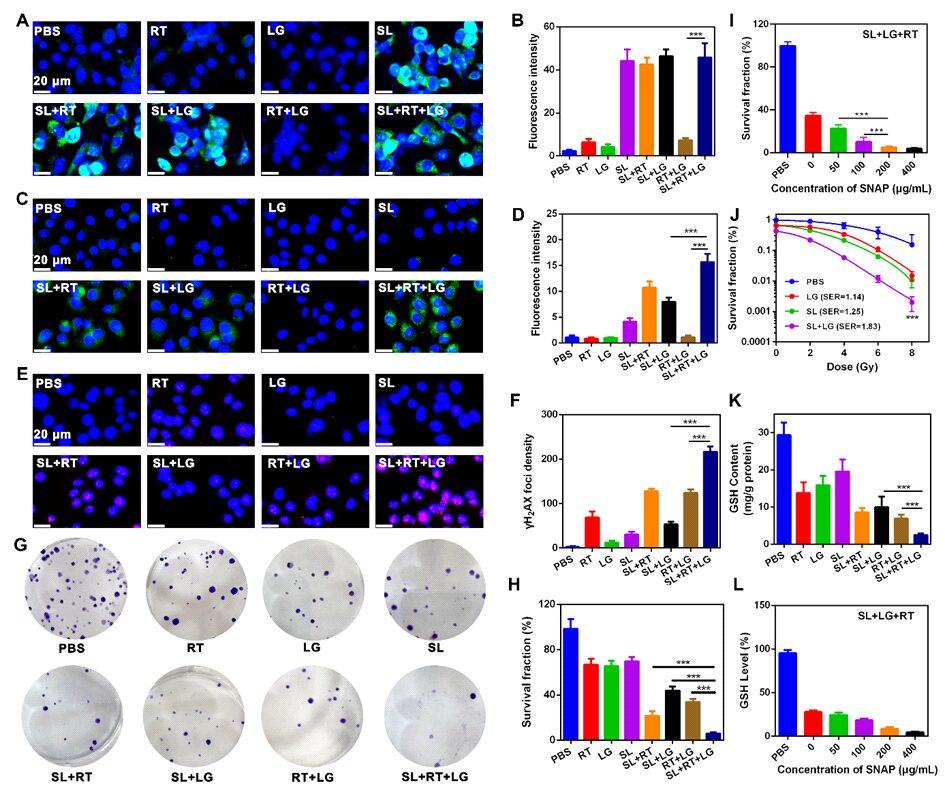
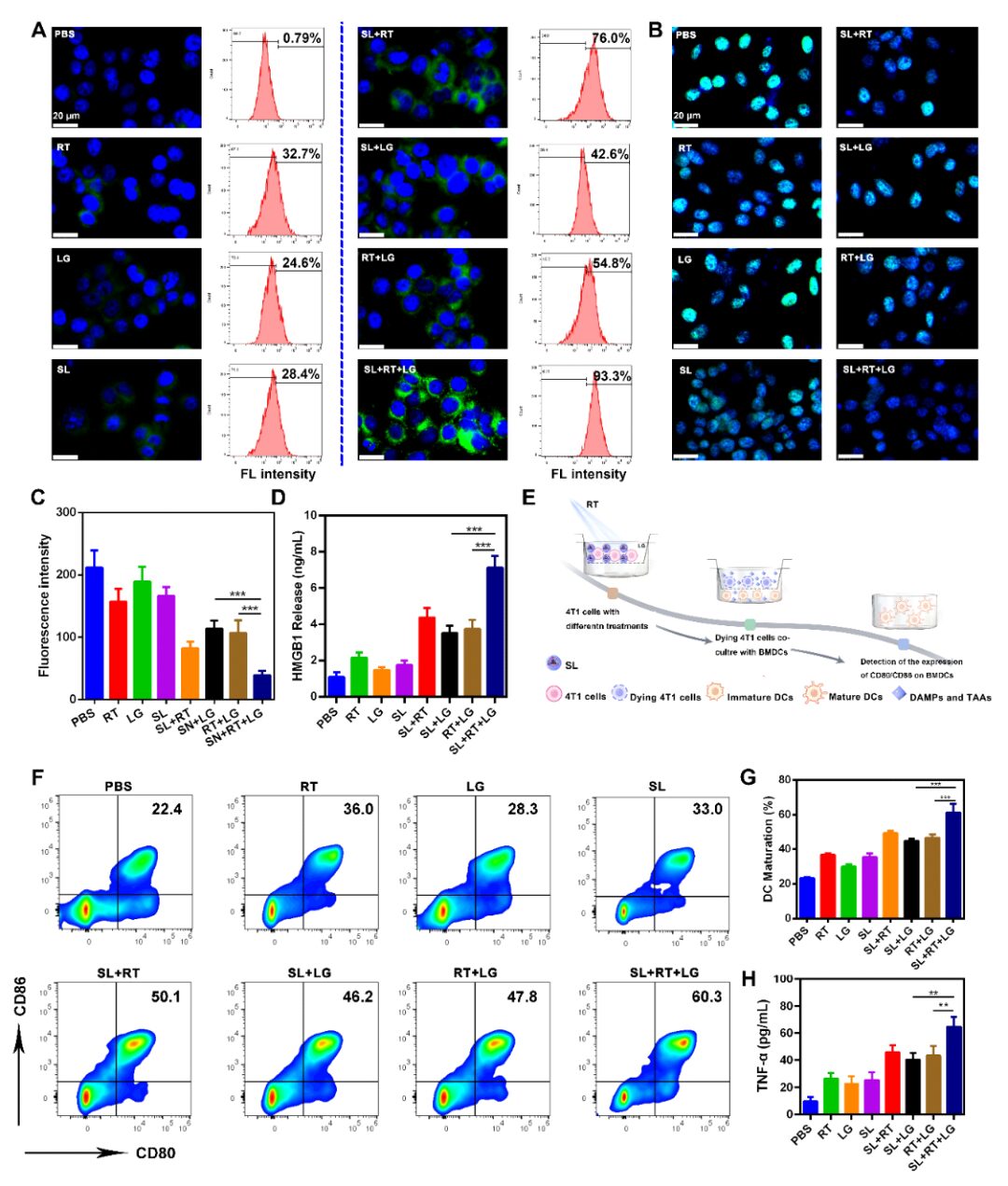
Cold exposure therapy (CE) is a low-cost, non-invasive treatment for malignant tumors. When the body is exposed to a cold environment, it activates the thermogenic metabolism of brown adipose tissue (BAT), quickly inducing tumor starvation and inhibiting tumor growth. The microenvironmental changes caused by CE can further complement conventional treatments (such as radiotherapy and chemotherapy), enhancing the effectiveness of traditional therapies. Currently, there are no research reports on CE combined treatment, thus researchers are urgently needed to explore the combined antitumor mechanisms of CE with traditional therapies (such as radiotherapy).On October 14, 2024, Professor Ning Zhipeng from Guangxi Medical University and Academician Chen Xiaoyuan from the National University of Singapore jointly communicated in the top international journalACS Nano (IF=15.8) with the title “Cold Exposure Therapy Sensitizes Nanodrug-mediated Radioimmunotherapy for Breast Cancer” published related research papers.



—END—
The content is original from 【iNature】,
Please indicate the source as 【iNature】
Add WeChat Group
iNature gathers 40,000 researchers and doctors in life sciences. We have established 80 comprehensive groups (16 PI groups and 64 PhD groups), and also formed specialized groups in relevant fields (plants, immunology, cells, microbiology, gene editing, neuroscience, chemistry, physics, cardiovascular, oncology, etc.).Warm reminder: Please note when joining the group (format: school + major + name, if you are a PI/professor, pleaseindicate as PI/professor, otherwise, it will be directlyassumed as a PhD student, thank you).You can first add the editor’s WeChat ID (love_iNature), or long press the QR code to add the editor, and then join the relevant group, serious inquiries only.


Submission, cooperation, and reprint authorization matters
Please contact WeChat ID:13701829856 or email:[email protected]
If you find this article interesting, please click here!