Bacterial infections pose a significant threat to global public health, causing approximately one million deaths each year. Currently, antibiotic treatment remains the core strategy for managing bacterial infections. However, the long-term use of antibiotics exacerbates the development of bacterial resistance, even leading to the emergence of multidrug-resistant (MDR) strains. Therefore, the development of alternative antibacterial strategies has become an urgent need.
Early detection and timely treatment are crucial for controlling bacterial infections, effectively reducing the risk of disease progression and mortality. However, the diagnosis of many bacterial infections still faces challenges, often requiring confirmation only after severe inflammatory responses occur, which can lead to difficult-to-treat complications. Traditional in vivo diagnostic methods, such as white blood cell counts, erythrocyte sedimentation rates, and C-reactive protein tests, suffer from insufficient sensitivity and specificity, and struggle to provide real-time information about the infection site. Therefore, there is an urgent need to develop an integrated diagnostic and therapeutic platform that can accurately locate the infection site and assess the severity of the infection in real-time, enabling efficient localized antibacterial treatment.

To address the above issues, the team led by Li Wen from the Chinese Academy of Medical Sciences developed a pH-responsive photodynamic probe TI, which was assembled with a reactive carbon monoxide (CO) donor that generates reactive oxygen species (ROS), and then encapsulated in a hyaluronic acid (HA)-based microneedle patch, ultimately forming a therapeutic microneedle platform. The microneedle structure enhances the mechanical penetration of the molecular probe and CO donor into the biofilm at the wound infection site. When encountering the acidic microenvironment of the wound, TI undergoes dynamic molecular structural changes, resulting in significant near-infrared fluorescence output for detecting the infection and assessing its severity. Meanwhile, the nanoprobe can also release its ROS-generating potential, which not only directly kills bacteria through oxidative damage but also triggers the release of CO for auxiliary gas therapy. In summary, the integrated diagnostic and therapeutic microneedle platform provides a promising approach to address the challenges of wound infections and mitigate the issues posed by antibiotic resistance, bringing significant hope to the field of infection care management. This article was published on December 12, 2024, under the title “Bacterial Microenvironment-Responsive Microneedle Patches for Real-Time Monitoring and Synergistic Eradication of Infection” in “Advanced Functional Materials” (DOI: 10.1002/adfm.202414834).
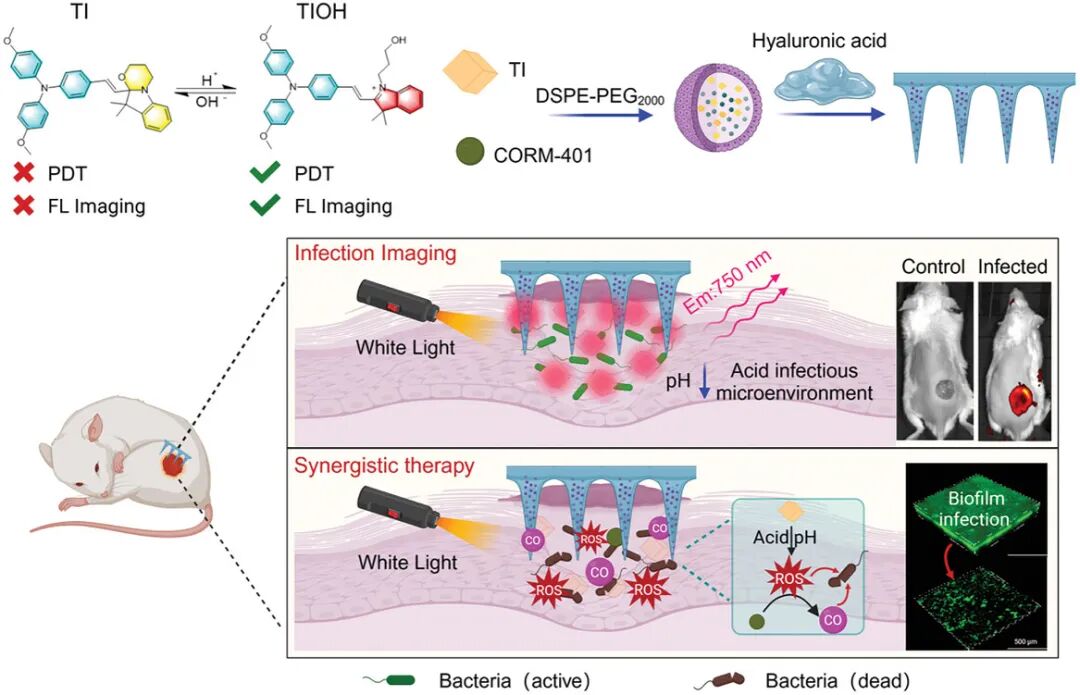
Figure 1. Schematic of the study
(1) Synthesis of pH-triggered structurally variable molecule TI and its photophysical properties study
Figure 2 (a) Schematic of the synthetic pathway showing the synthesis steps of the target molecule (TI) and the formation of key intermediates. Figure 2 (b) Structural transformation mechanism illustrating the structural changes of TI under different pH conditions: under alkaline conditions, TI presents a closed-loop structure, while under acidic conditions, the ring opens to form a D-A structure with strong electron-accepting characteristics. Figure 2 (c) shows the NMR spectra of TI under different pH conditions, with new peaks appearing in acidic environments, indicating molecular structural changes. Figure 2 (d) The absorption spectra show the UV-Vis absorption spectra of TI at different pH values, with the absorption peak at 550 nm increasing as pH decreases, indicating structural transformation. Figure 2 (e) The pKa value of TI was calculated to be approximately 6.39 based on the absorbance change curve at 550 nm, suitable for detecting weakly acidic infection environments. Figure 2 (f) The fluorescence spectra show that the fluorescence intensity of TI increases as pH decreases, exhibiting a significant “off-to-on” NIR fluorescence response. Figure 2 (g) Fluorescence imaging shows the fluorescence images of TI under different pH conditions, with significantly enhanced fluorescence signals in acidic environments. Figure 2 (h) After cycling TI between pH 8 and pH 3 five times, the absorbance signal showed no significant decay, proving its reversibility and stability. Figure 2 (i) Under light exposure, the absorbance of TIOH remains almost unchanged, while ICG rapidly decays, indicating that TIOH has excellent photostability. Figure 2 (j) Selectivity tests show that TI has high selectivity for pH in the presence of various biologically relevant substances, with minimal interference. Overall, TI exhibits excellent pH-dependent fluorescence response, good stability, and high selectivity, making it an ideal probe for infection diagnosis and treatment.
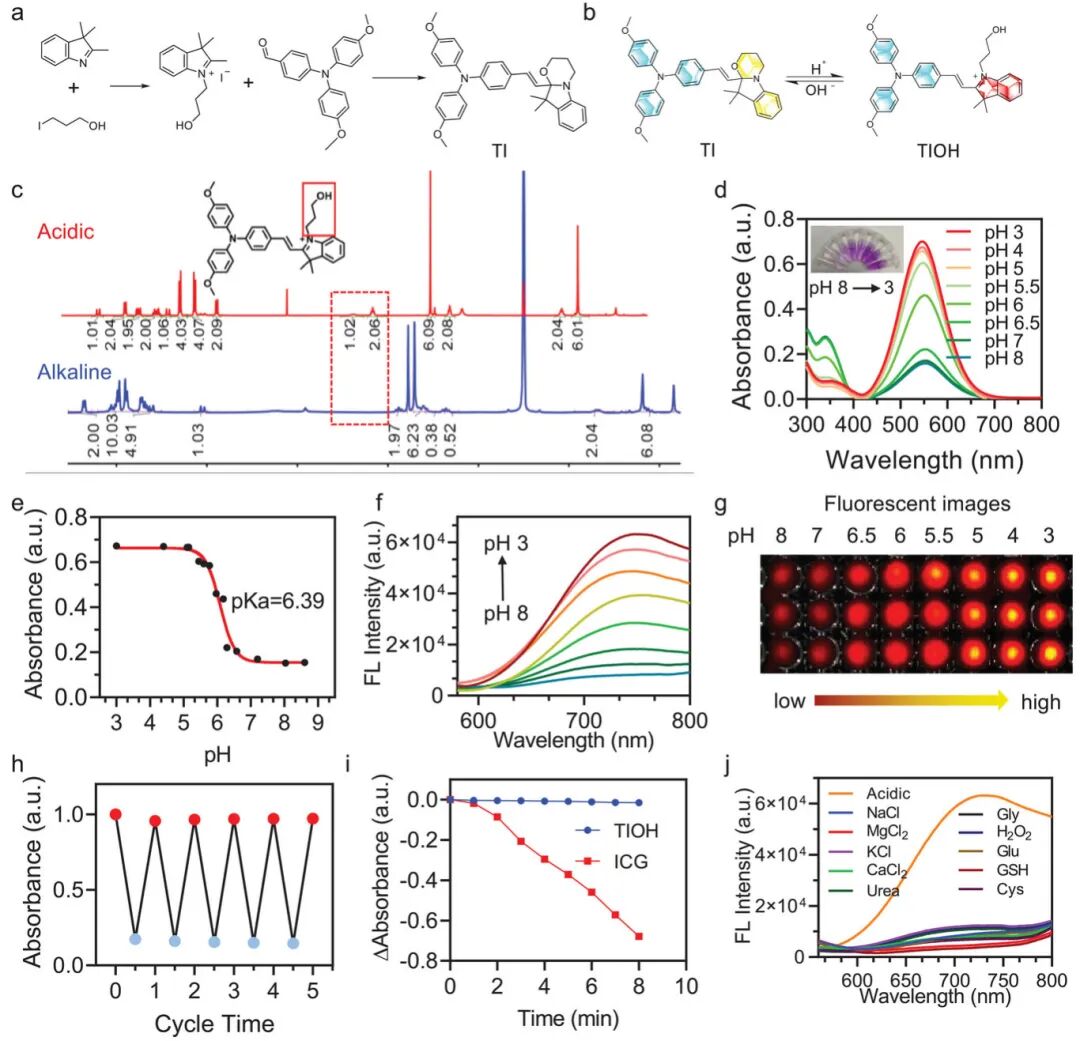
Figure 2. Synthesis of pH-triggered structurally variable molecule TI and its photophysical properties study.(a) Synthetic route of TI; (b) pH-responsive structural transformation of TI; (c) ¹H NMR of TI under acidic and alkaline conditions; (d) Absorbance and solution color of TI at different pH values; (e) pKa obtained from titration fitting curve; (f) Fluorescence spectra of TI at different pH values; (h) Reversible absorbance of TI molecules switching between pH 3 (red circle) and pH 8 (blue circle) over five cycles; (i) UV absorption changes of ICG and TIOH after 8 minutes of white light (0.1 W cm⁻²) exposure; (j) Fluorescence changes of TI in the presence of different biological interferents.
(2) Study of photodynamic effects of TI under different pH conditions
Figure 3a shows the ability of TI molecules to generate reactive oxygen species (ROS) under light exposure in acidic conditions. As the light exposure time increases, the absorbance of DPBF at 412 nm significantly decreases, indicating ROS generation. Figure 3b shows the absorbance changes of the mixture of TI and DPBF under light exposure at different pH conditions, with TI effectively generating ROS only under acidic conditions (pH 5). Figure 3c shows the ability of TI molecules to produce singlet oxygen (¹O₂) under light exposure in acidic conditions, with the absorbance of ABDA rapidly decreasing under light exposure, indicating ¹O₂ generation. Figure 3d shows the ability of TI molecules to produce hydroxyl radicals (•OH) under light exposure in acidic conditions, with the absorbance of TMB significantly increasing under light exposure, confirming •OH generation. Figure 3e confirms the ability of TI molecules to produce ¹O₂ and •O₂⁻ under light exposure through electron spin resonance (ESR) spectroscopy, using TEMP and DMPO as spin traps, detecting characteristic signals of ¹O₂ and •O₂⁻, respectively. Figure 3f shows the energy level structure of TI and acid-activated TIOH, with theoretical calculations indicating that acid-activated TIOH has a smaller bandgap (2.61 eV), facilitating a more efficient intersystem crossing (ISC) process, thereby enhancing ROS generation. These figures collectively demonstrate that TI molecules exhibit significant ROS generation capabilities under acidic conditions, including ¹O₂ and •OH, and this capability can be triggered by light exposure. Furthermore, acid-activated TIOH, due to its smaller bandgap, shows a more efficient ISC process, enhancing ROS generation. These characteristics make TI a promising candidate for photodynamic therapy (PDT) for precise and efficient infection treatment.
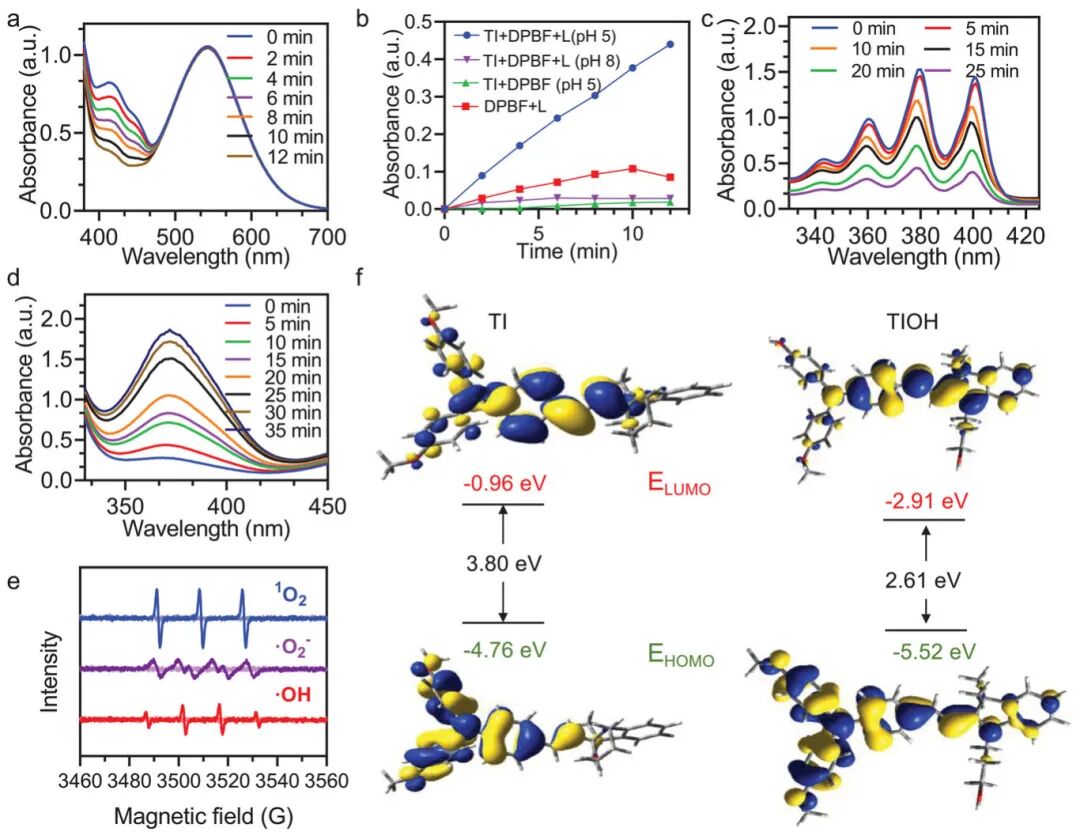
Figure 3. Study of photodynamic effects of TI under different pH conditions.(a) Changes in absorbance of DPBF under white light exposure (0.1 W cm⁻²) in the presence of TI at acidic pH (pH = 5); (e) Confirmation of TI’s type I and II photodynamic therapy (PDT) effects through electron spin resonance (ESR) spectroscopy; (f) Calculated molecular geometries, highest occupied molecular orbitals (HOMO), lowest unoccupied molecular orbitals (LUMO), and energy levels of TI (original form) and TIOH (acidic form).
(3) Study of the photophysical properties and biocompatibility of TI nanoparticles
Figure 4a shows the particle size distribution and transmission electron microscopy (TEM) images of TI nanoparticles (NPs). Dynamic light scattering (DLS) data show an average particle size of 126 nm, with a polydispersity index (PDI) of 0.15, and TEM images confirm the uniform spherical structure of the particles, with an average diameter of approximately 110 nm. Figure 4b shows the UV-visible absorption spectra of TI nanoparticles at different pH values. As the pH decreases, the absorption intensity of TI nanoparticles at 342 nm gradually weakens, while a new absorption peak appears at 550 nm, indicating their pH-responsive photophysical properties. Figure 4c shows the ability of TI nanoparticles to generate reactive oxygen species (ROS) under light exposure in acidic conditions. As the light exposure time increases, the absorbance of DPBF gradually decreases, indicating that TI nanoparticles can effectively generate ROS. Figure 4d shows the ability of TI nanoparticles to release carbon monoxide (CO) under light exposure in acidic conditions. As the light exposure time increases, the fluorescence intensity of the CO probe significantly increases, indicating that TI nanoparticles can control the release of CO. Figure 4e shows the effect of TI nanoparticles on the cell viability of L929 mouse fibroblasts. The results indicate that TI nanoparticles exhibit low toxicity to cells at different concentrations, with cell viability remaining above 86% even under light exposure. Figure 4f shows the impact of TI nanoparticles on cell structure through cytoskeletal staining experiments. The results show that the treated cells exhibit no significant changes in structure, indicating that TI nanoparticles have minimal impact on cell structure. Figure 4g shows the effect of TI nanoparticles on cell viability through live/dead cell co-staining experiments. The results show that the treated cells primarily exhibit green fluorescence (live cells), with red fluorescence (dead cells) being rare, indicating that TI nanoparticles have low toxicity to cells. These figures collectively demonstrate that TI nanoparticles possess good biocompatibility, pH-responsive photophysical properties, and the ability to generate ROS and release CO under acidic conditions, making them a promising antibacterial nanomaterial.
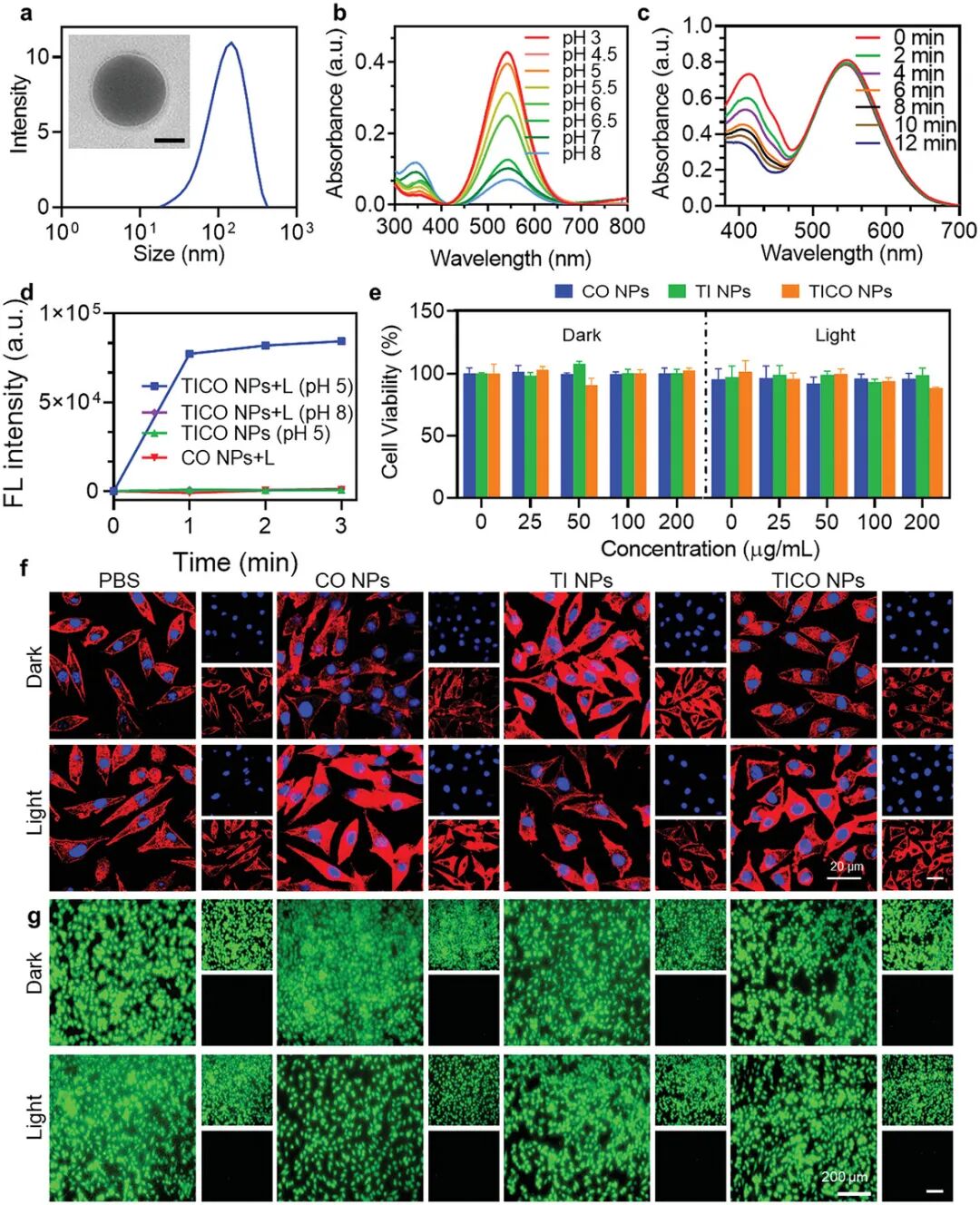
Figure 4. Study of the photophysical properties and biocompatibility of TI nanoparticles.(a) DLS and TEM analysis of TI NPs; (b) Absorbance of TI NPs at different pH values; (c) Changes in absorbance of DPBF exposed to TICO NPs + white light (0.1 W cm⁻²) under acidic pH; (d) Fluorescence intensity of the CO probe at its characteristic peak of 515 nm, indicating the release level of CO in different systems; (f) Cytoskeletal staining; (g) Live/dead staining images of L929 cells under different treatments, scale bar = 200 µm.
(4) Evaluation of the antibacterial ability of TICO nanoparticles against Escherichia coli and Staphylococcus aureus
Figures 5a and 5b show the effects of different treatments (PBS, CO NPs, TI NPs, TiCO NPs) on the growth of Staphylococcus aureus (S. aureus) and Escherichia coli (E. coli) on agar plates under dark and light conditions. It can be observed that only TiCO NPs significantly inhibited bacterial growth under light conditions. Figure 5c shows the logarithmic values of colony-forming units (CFU/mL) of S. aureus and E. coli under different treatment conditions. The results indicate that TiCO NPs exhibit strong bactericidal effects against both bacteria under light conditions, achieving bactericidal efficiencies of approximately 98.3% and 99.9%, respectively. Figures 5d and 5e show the morphological changes of bacteria under different treatment conditions observed through scanning electron microscopy (SEM). It can be seen that the bacterial cell walls treated with TiCO NPs under light conditions suffered severe damage, with extensive rupture and collapse, while the bacterial structures treated with TiCO NPs alone or light treatment remained relatively intact. Figures 5f and 5g show the survival status of bacteria under different treatment conditions through live/dead cell co-staining experiments. The results indicate that the bacteria treated with TiCO NPs under light conditions primarily exhibit red fluorescence, indicating a high bactericidal effect. Overall, these results demonstrate that TiCO nanoparticles exhibit significant bactericidal effects against both S. aureus and E. coli under light conditions. This effect is primarily attributed to the synergistic action of photodynamic therapy (PDT) and carbon monoxide (CO), which together disrupt the bacterial cell wall and membrane, ultimately leading to the contraction or even rupture of the bacterial cell membrane. These findings provide strong evidence for TiCO nanoparticles as an effective antibacterial agent.
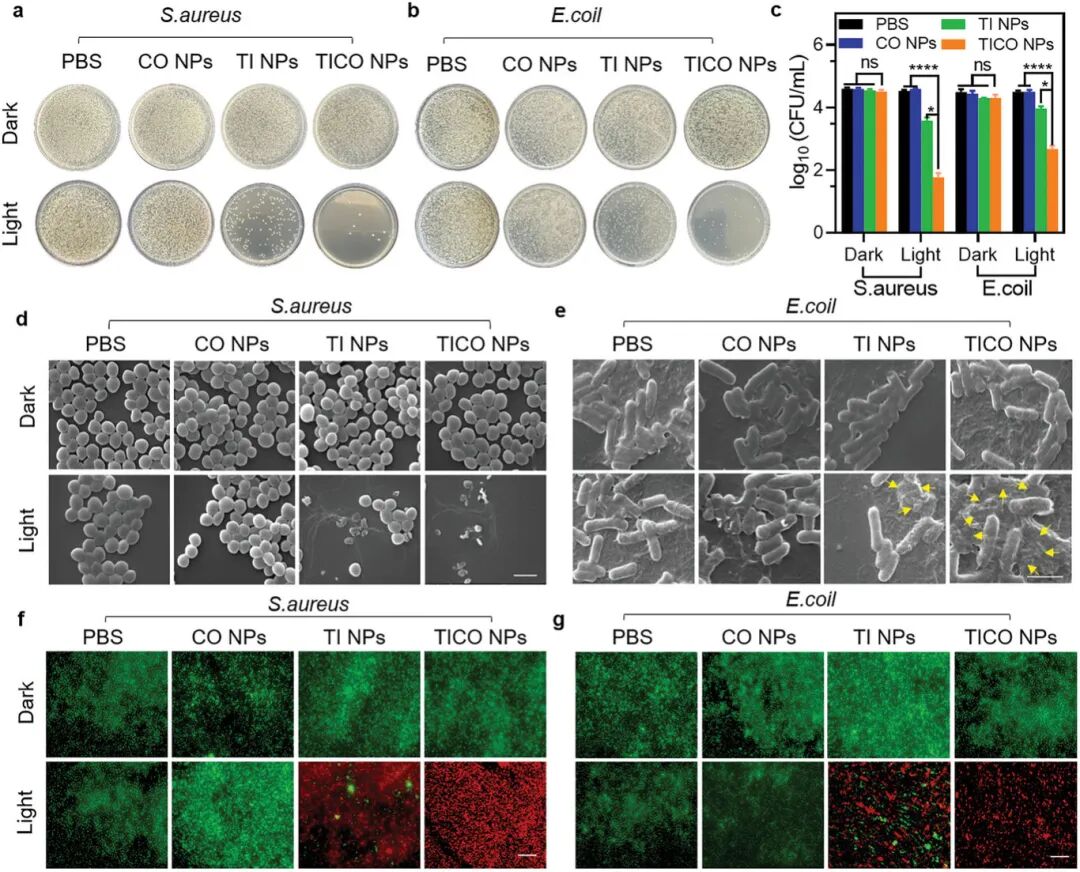
Figure 5. Evaluation of the antibacterial ability of TICO nanoparticles against Escherichia coli and Staphylococcus aureus.(a) Representative photos of Staphylococcus aureus on plates after different treatments; (b) Representative photos of Escherichia coli on plates after different treatments; (d) Representative scanning electron microscopy images of Staphylococcus aureus after different treatments (scale bar = 2 micrometers); (e) Representative scanning electron microscopy images of Escherichia coli after different treatments (scale bar = 2 micrometers); (f) Live (green fluorescence)/dead (red fluorescence) staining images of Staphylococcus aureus under different groups, scale bar = 100 micrometers; (g) Live (green fluorescence)/dead (red fluorescence) staining images of Escherichia coli under different groups, scale bar = 100 micrometers. Samples in the light group were exposed to white light (0.1 W cm⁻²) for 10 minutes.
(5) Preparation of TICO@MN patches and their mechanical properties and biocompatibility study
Figure 6a shows the preparation process of the microneedle (MN) patches. First, TiCO nanoparticles were mixed with high molecular weight hyaluronic acid (HMHA) to form a pre-hydrogel solution, which was then poured into polydimethylsiloxane (PDMS) molds, followed by vacuum degassing and drying. Next, low molecular weight hyaluronic acid (LMHA) was coated on the surface of the microneedles, centrifuged to form an independent layer, and finally peeled off from the mold after drying. Figures 6b and 6c show the morphology of the microneedles observed through scanning electron microscopy (SEM). The prepared TiCO@MN exhibits orderly arranged 10×10 pyramidal microneedles, each with a height of 1000 µm, a base width of 500 µm, and a center-to-center distance of 550 µm. Figure 6d shows the separation process of the microneedles. By loading rhodamine B (RhB) dye into the microneedles and immersing the microneedle patch in PBS solution, the separation of the microneedles from the backing layer was observed. Figure 6e shows the real-time observation of the separation state of the microneedles after contact with the aqueous solution through optical microscopy. The results indicate that the microneedles rapidly separate from the backing layer upon contact with the aqueous solution, completing separation within 2 minutes. Figure 6f shows the mechanical strength test results of TiCO@MN. Through compression testing, TiCO@MN exhibited mechanical strength similar to that of hyaluronic acid microneedles (HA MN), with a compression force of approximately 2.3 N per microneedle, sufficient to penetrate the stratum corneum barrier. Figure 6g shows the penetration ability of TiCO@MN on fresh pig skin. By applying thumb pressure, the microneedle patch can easily insert into the skin, leaving clear and neat puncture holes, indicating its excellent skin penetration ability. Figure 6h further confirms the penetration of microneedles through the stratum corneum and into the epidermis with hematoxylin and eosin (H&E) staining, with a penetration depth of approximately 150 µm. Figure 6i shows the cell compatibility test results of TiCO@MN. Cell viability was assessed using the CCK-8 method, and the results indicate that TiCO@MN has high cell compatibility. In summary, TiCO@MN patches combine the advantages of mechanical penetration and controlled release, showing great potential for on-demand transdermal infection management.
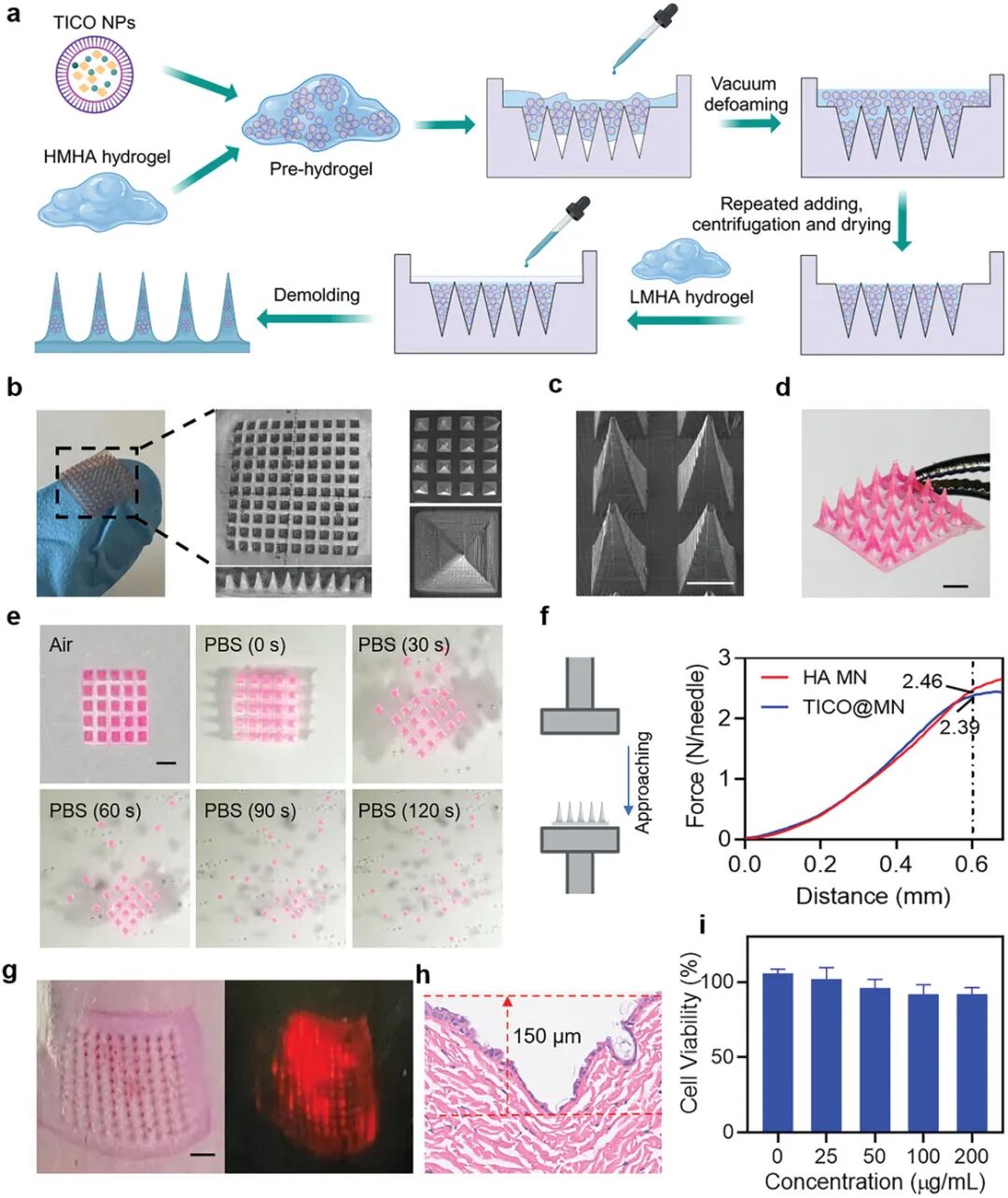
Figure 6. Preparation of TICO@MN patches and their mechanical properties and biocompatibility study.(a) Schematic of the preparation process of TICO@MN patches: mixing TICO NPs with high molecular weight hyaluronic acid (HMHA) solution, pouring into PDMS molds, repeated vacuum degassing and drying; (b) Optical image of TICO@MN; (c) Scanning electron microscopy image of TICO@MN; (d) Optical image of MN patches loaded with rhodamine B (RhB); (e) Optical image showing the separation of MN bodies from the backing layer after being cultured in PBS for a period; (f) Measurement of mechanical compression strength of HA MN and TICO@MN patches; (g) Representative photos and fluorescence images of mouse skin after applying MN patches containing RhB; (h) Hematoxylin-eosin (H&E) staining images of mouse skin after TICO@MN treatment; (i) Assessment of the cytotoxicity of TICO@MN on L929 mouse fibroblasts under light exposure using the CCK-8 method.
(6) Study of pH-responsive sensing and antibacterial performance of TICO@MN patches
Figure 7a shows the fluorescence intensity of TiCO nanoparticles in gels under different pH conditions. The results indicate that in acidic environments (pH 3-5), TiCO nanoparticles exhibit strong near-infrared (NIR) emission, while in alkaline or neutral environments (pH 6-9), the fluorescence intensity is weak. This indicates that TiCO nanoparticles have good pH responsiveness. Figure 7b shows the biomass of Staphylococcus aureus (S. aureus) biofilm after crystal violet staining under different treatment conditions. It can be seen that the biomass of biofilm treated with TiCO@MN under light conditions is significantly reduced, indicating its excellent anti-biofilm effect. Figure 7c quantitatively analyzes the biomass of biofilm under different treatment conditions through crystal violet staining. The results show that the biomass of biofilm treated with TiCO@MN under light conditions decreased by approximately 60%, significantly higher than other treatment groups. Figure 7d shows the 3D reconstruction staining of live bacteria in biofilms using confocal laser scanning microscopy (CLSM). It can be seen that the biofilm treated with TiCO@MN under light conditions has significantly reduced live bacteria, with weak green fluorescence. Figure 7e quantitatively analyzes the proportion of live bacteria in biofilms under different treatment conditions. The results show that the proportion of live bacteria in biofilms treated with TiCO@MN under light conditions is only about 9.7%, significantly lower than other treatment groups. In summary, TiCO@MN patches exhibit excellent disruption and bactericidal effects against Staphylococcus aureus biofilms under light conditions, primarily attributed to the synergistic action of photodynamic therapy (PDT) and carbon monoxide (CO) gas therapy. These results indicate that TiCO@MN patches have good application prospects in anti-infection treatment, especially in combating biofilm infections.
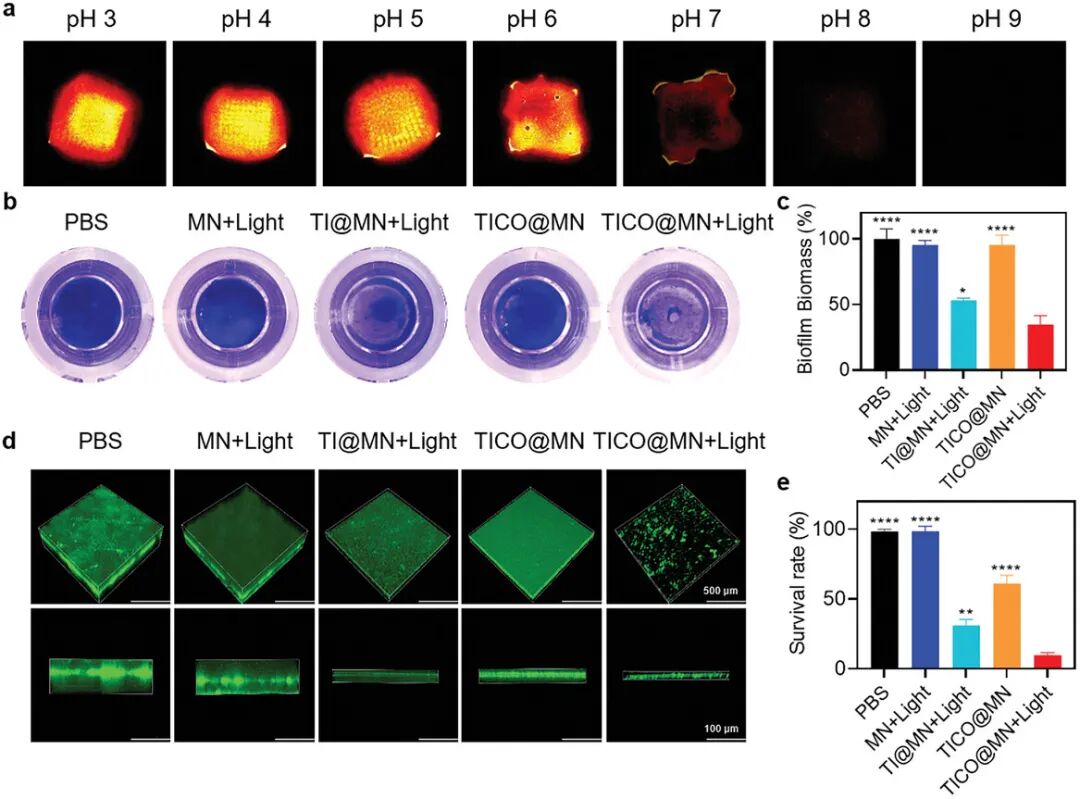
Figure 7. Study of pH-responsive sensing and antibacterial performance of TICO@MN patches.(a) Near-infrared fluorescence images of TICO@MN patches applied to gels of different pH values observed under IVIS (in vivo imaging system); (b) Representative photos of biofilms stained with crystal violet under different treatments; (d) Three-dimensional confocal images of Staphylococcus aureus (SA) biofilms stained with SYTO9 after different treatments, scale bar = 500 µm; (e) Quantitative assessment of bacterial survival rates in SA biofilms under different groups based on the staining images described in (d), data presented as mean ± standard deviation (SD) (n = 3 independent experimental repeats). Samples in the light group were exposed to white light (0.1 W cm⁻²) for 10 minutes.
(7) Evaluation of the ability of TICO@MN to monitor pH changes at the infection site in vivo
Figure 8a shows the experimental process for real-time monitoring of pH changes in a mouse infection model. First, a circular wound with a diameter of 8 mm was created on the back skin of BALB/c mice, followed by inoculation with Staphylococcus aureus (S. aureus) suspension to induce infection. At time points of 2, 6, 12, and 24 hours post-bacterial inoculation, the TiCO@MN patch was implanted into the infected wound to assess its ability to monitor the infection microenvironment. Figure 8b shows the fluorescence imaging of the TiCO@MN patch at the mouse wound site at different time points (0, 2, 6, 12, 24 hours) post-infection. It can be seen that as the infection progresses, the red fluorescence signal emitted by the TiCO@MN patch gradually increases, peaking at 12 hours post-infection. Figure 8c quantitatively analyzes the fluorescence intensity of the TiCO@MN patch at different time points (0, 2, 6, 12, 24 hours). The results indicate that the fluorescence intensity at 12 hours post-infection is significantly higher than at other time points, indicating that the TiCO@MN patch can effectively monitor the progression of the infection. Figure 8d shows the fluorescence imaging of the TiCO@MN patch at the mouse wound site under different infection severities (1×10^6, 1×10^7, 1×10^8 CFU/mL). It can be seen that the higher the initial bacterial concentration, the stronger the near-infrared (NIR) fluorescence signal at the infection site. Figure 8e quantitatively analyzes the fluorescence intensity of the TiCO@MN patch under different infection severities. The results indicate that the higher the initial bacterial concentration, the greater the fluorescence intensity, indicating that the TiCO@MN patch can distinguish different infection severities. In summary, TiCO@MN patches exhibit excellent pH-responsive characteristics in in vivo experiments, effectively monitoring pH changes in the infection microenvironment and distinguishing different infection severities. These results indicate that TiCO@MN patches have significant potential for real-time monitoring of infection progression and assessing infection severity.
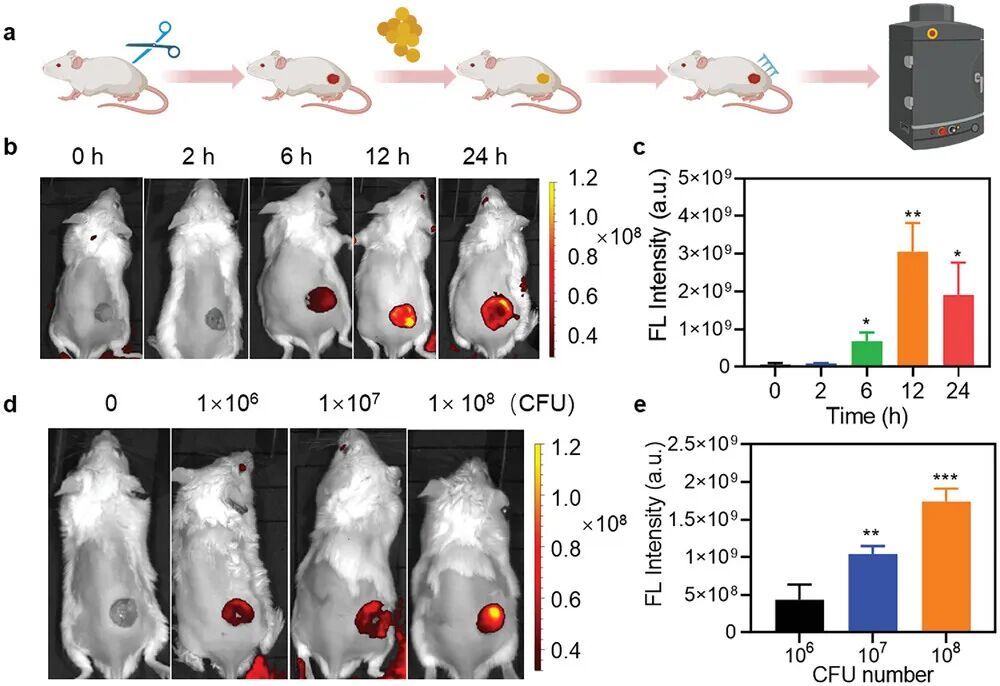
Figure 8. Evaluation of the ability of TICO@MN to monitor pH changes at the infection site in vivo.(a) Schematic of the process for creating bacterial wound infection models using TICO@MN and performing infection imaging; (b) Representative IVIS (in vivo imaging system) images of wounds treated with TICO@MN at different time points post-bacterial inoculation; (d) Representative IVIS images of wounds treated with TICO@MN at different severities, induced by challenge wounds with different bacterial loads; (e) Corresponding quantitative analysis of fluorescence intensity at the infection site based on the fluorescence images in (d).
(8) Evaluation of the antibacterial and wound healing abilities of TICO@MN in a mouse subcutaneous infection model
Figure 9a shows the experimental process for the mouse subcutaneous infection model. First, a wound was created on the back skin of the mouse, followed by inoculation with Staphylococcus aureus (S. aureus) to induce infection. Twelve hours post-infection, the infected mice were randomly divided into six groups for different treatments: 1) PBS, 2) light only, 3) TiCO@MN, 4) Ti@MN + light, 5) TiCO NPs + light, or 6) TiCO@MN + light. In cases involving light treatment, the wounds of the mice were exposed to white light at an intensity of 0.1 W/cm² for 10 minutes after administration. Figure 9b shows the progress of wound healing in different treatment groups during the treatment period. It can be seen that the TiCO@MN + light group exhibited the fastest wound healing rate and the best healing effect. Figure 9c quantitatively analyzes the wound closure rates of different treatment groups. The results indicate that the TiCO@MN + light group had the highest wound closure rate, reaching approximately 87%, significantly higher than other groups. Figure 9d shows the histological examination of wound tissues on day seven through H&E staining. It can be seen that the TiCO@MN + light group exhibited significantly reduced inflammatory responses, with wound infections nearly completely resolved, accompanied by fibroblasts and newly formed epithelial structures. Figure 9e shows the bacterial colony counts in different treatment groups. It can be seen that the TiCO@MN + light group significantly reduced bacterial colony counts, indicating its excellent bactericidal effect. Figure 9f quantitatively analyzes the bacterial colony counts in different treatment groups. The results indicate that the bacterial count in the TiCO@MN + light group decreased by 96.3%, significantly higher than other groups. Figure 9g analyzes the levels of IL-6 and TNF-α in infected tissues through enzyme-linked immunosorbent assay (ELISA). It can be seen that the levels of IL-6 and TNF-α in the TiCO@MN + light group were significantly lower than those in other groups, indicating its ability to effectively suppress inflammatory responses. Figure 9h quantitatively analyzes the levels of IL-6 in different treatment groups. The results indicate that the IL-6 levels in the TiCO@MN + light group were the lowest, further confirming its anti-inflammatory effects.
In summary, TiCO@MN patches exhibit excellent antibacterial and wound healing abilities in a mouse subcutaneous infection model. The TiCO@MN + light group not only effectively clears infections but also modulates the local immune environment, promoting tissue regeneration. These results confirm the potential and good biosafety of TiCO@MN patches in infection imaging and antibacterial treatment.
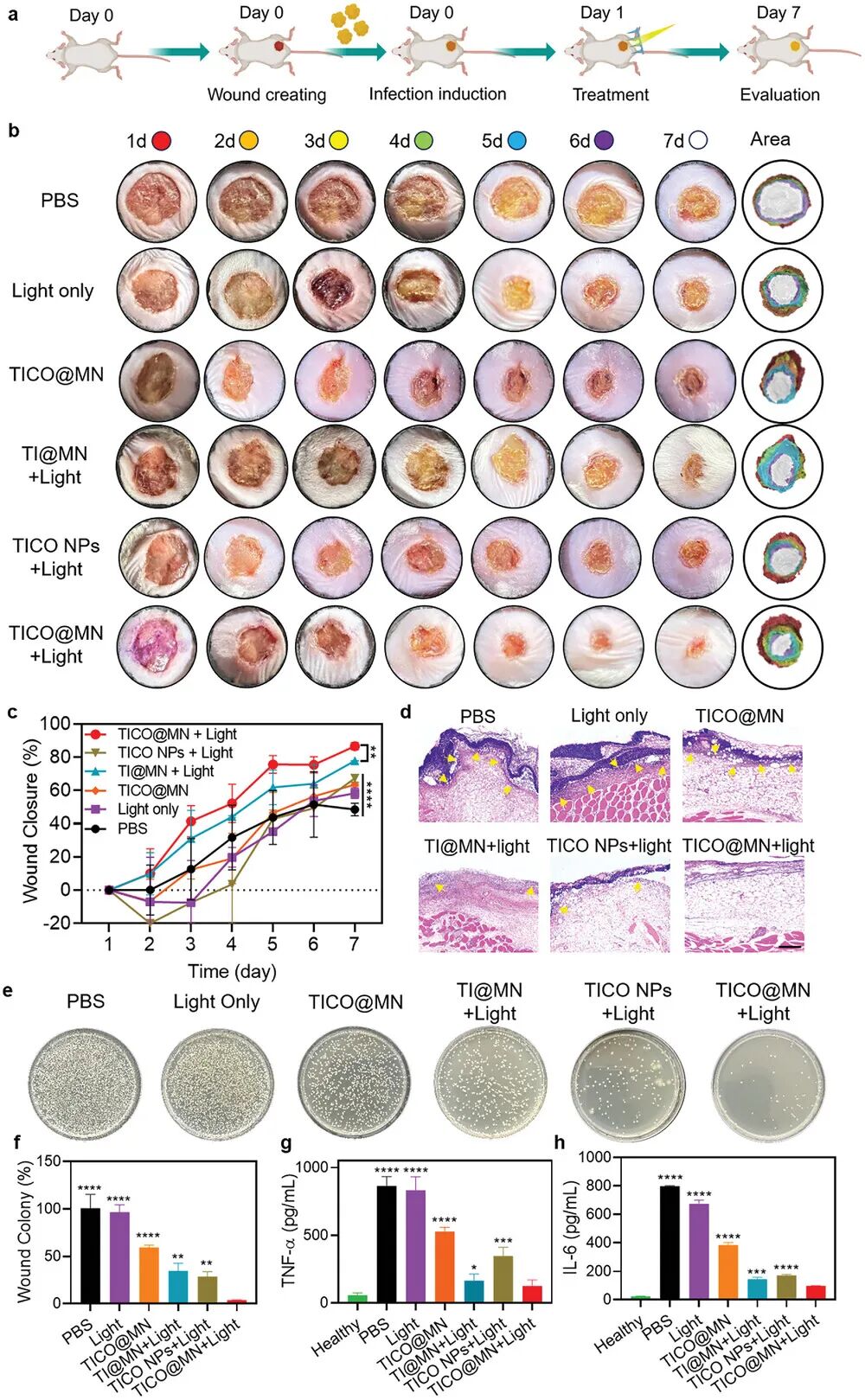
Figure 9. Evaluation of the antibacterial and wound healing abilities of TICO@MN in a mouse subcutaneous infection model.(a) Schematic of the evaluation process of the antibacterial and wound healing abilities of TICO@MN in vivo; (b) Representative photos of mouse wounds at different time points after different treatments; (c) Quantitative analysis of wound healing rates of different groups at different time points; (d) Representative hematoxylin-eosin (H&E) staining images of wound tissues on day 7 from different groups, with yellow arrows indicating inflammatory cell infiltration; (e) Representative photos of bacterial culture plates from wound tissues of different groups; (f) Quantitative analysis of CFU (colony-forming units) counts corresponding to the results in (e) from different groups; (g) ELISA analysis of TNF-α levels in mouse wounds after different treatments; (h) ELISA analysis of IL-6 levels in mouse wounds after different treatments. Data are presented as mean ± standard deviation (SD) (n = 3 mice). Samples in the light group were exposed to white light (0.1 W cm⁻²) for 10 minutes.
「 Research Summary 」
This study developed a therapeutic microneedle (MN) platform that combines real-time imaging activated by the infection microenvironment with on-demand bacterial elimination functions. A high-performance phototherapy diagnostic organic molecular probe named TI was designed and synthesized, featuring weakly acidic pH responsive near-infrared (NIR) fluorescence emission and reactive oxygen species (ROS generation capabilities. The hydrophobic probe TI was co-assembled with a ROS-responsive carbon monoxide (CO) donor to form an efficient bacterial nanokiller, encapsulated in hyaluronic acid (HA) based microneedles.
The microneedle patches enhance the mechanical penetration of TiCO nanoparticles at the subcutaneous infection site and degrade under the action of bacterial-secreted hyaluronidase to release the nanoparticles. When encountering acid-producing bacteria, TiCO nanoparticles undergo dynamic molecular structural changes, producing significant fluorescence output that can accurately detect infections in real-time and distinguish their severity. Meanwhile, the nanoprobe releases ROS-generating capabilities, directly killing bacteria through oxidative damage and promoting CO release for gas therapy. The cascade-activated photodynamic therapy (PDT)/CO gas therapy achieved powerful bacterial elimination effects in both in vitro cell and subcutaneous wound infection mouse models. Furthermore, TiCO@MN combined with light treatment can alleviate wound inflammatory responses and accelerate wound healing.
This intelligent microneedle system provides real-time infection monitoring and on-demand, targeted treatment, offering a promising approach to address wound infection issues while mitigating the challenges posed by antibiotic resistance. This research advances the methods of precise infection diagnosis and treatment, laying the foundation for the development of intelligent home healthcare systems and providing significant hope for infection care and management.
Original link:
https://doi.org/10.1002/adfm.202414834
Click the left bottom corner at the end of the article “Read the original” to quickly jump to the original literature
👇 Click to follow the public account for more academic frontiers 👇
Previous exciting recommendations
IF: 18.5 “AFM” Gu Zhipeng from Sichuan University: Effective injectable hydrogels for treating hemophilic arthritis through anti-inflammatory, iron removal, and cartilage protection
IF: 18.5 “AFM” Peng Xin from Central South University, Shan Hong, and Liao Weihua from Xiangya Hospital: Stage-transformation hydrogels triggered by blood microenvironments for precise vascular embolization
IF: 27.4 “AM” Morphology-switchable Au nanowires and hemoglobin-resveratrol nanoparticles co-delivered in microneedles for diabetic wound healing treatment
IF: 18.5 “AFM” Pan Jingye from Wenzhou Medical University: Carbon nanotube-enhanced carbonized cellulose aerogels improve hemostasis and accelerate skin wound healing
IF: 13.4 “CEJ” Fan Hongsong from Sichuan University: Magnetic switching longitudinal matrix mechanical vibration enhances battery clutch engagement and Ca2+ influx promotes BMSCs neural differentiation and TBI repair
👉 Contact us


Address: No. 3, Juquan Road, Huangpu District, Guangzhou International Business Incubator Room A702
Phone:020-3202 9909
Website:www.chuangseed.com
