




This study explores the application of pure organic nanoparticles in tumor therapy, particularly their potential in photothermal radiotherapy combined treatment. The background of the article points out that the hypoxic state of the tumor microenvironment is a significant reason for radiotherapy resistance, while photothermal therapy can improve the hypoxic state and enhance the radiotherapy effect through thermal damage and immunogenic cell death. The innovation of the article lies in the design of a pure organic nanoparticle with aggregation-induced emission characteristics, which exhibits good photothermal conversion efficiency, biocompatibility, and tumor targeting ability. Experimental results show that this nanoparticle demonstrates significant anti-tumor effects in photothermal radiotherapy combined treatment, effectively killing tumor cells and activating immune cells. Furthermore, this nanoparticle also has good biocompatibility, with no significant toxic side effects observed in in vivo experiments. This research provides new ideas for tumor treatment and is expected to promote the clinical application of pure organic photothermal agents and radiosensitizers.

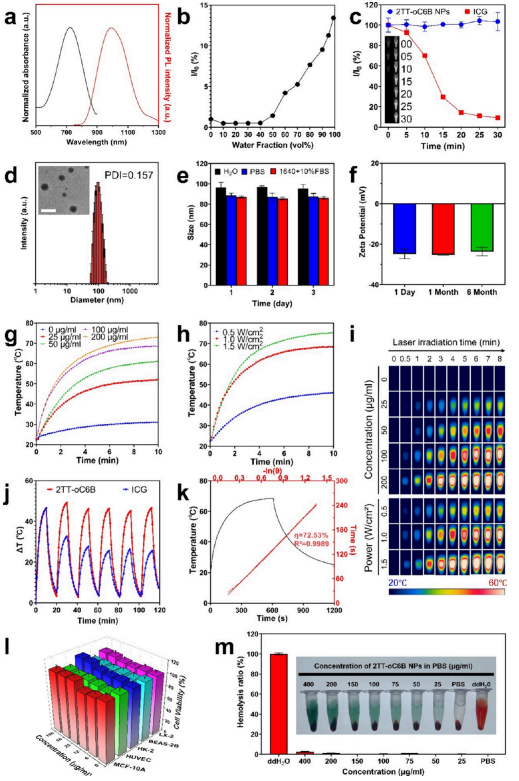
Figure 1. Characterization and properties of 2TT-oC6B nanoparticles. a) Absorption and emission spectra of 2TT-oC6B nanoparticles. b) αAIE curve with varying water fractions. c) Photostability curves of 2TT-oC6B nanoparticles and ICG after 30 minutes of continuous laser irradiation (808 nm, 1 W/cm2). Insets show NIR-II fluorescence images of 2TT-oC6B nanoparticles (left) and ICG (right) at different time points. d) Size distribution of 2TT-oC6B nanoparticles in water. TEM images (inset) show 2TT-oC6B nanoparticles. Scale bar: 200 nm. e) Size stability analysis of 2TT-oC6B nanoparticles in H2O, PBS, and RPMI 1640 + 10% FBS at 25°C. f) Zeta potential of 2TT-oC6B nanoparticles at different time points. g) Photothermal time-temperature curves at different concentrations. h) Photothermal time-temperature curves at different powers. i) Thermal imaging camera recordings. j) Heating curves of photothermal stability tests (six laser switch cycles, 1 W/cm2). k) PCE heating curves and calculated results. l) Cell viability of MCF-10A, HUVEC, HK-2, BEAS-2B, and LX-2 cell lines after incubation with different concentrations of 2TT-oC6B nanoparticles for 24 hours. m) Hemolysis test of 2TT-oC6B nanoparticles.
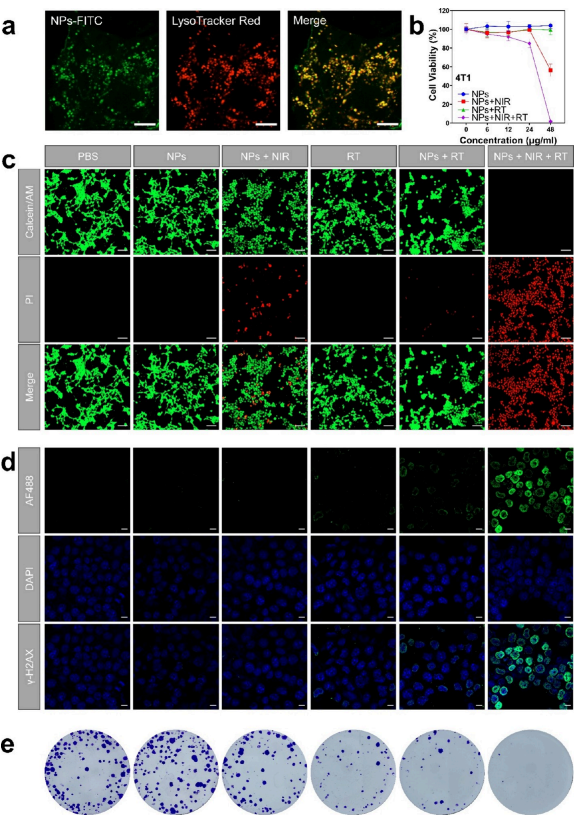
Figure 2. In vitro cell experiments combining photothermal therapy (PTT) with radiotherapy (RT) using 2TT-oC6B nanoparticles. a) Confocal laser scanning microscopy (CLSM) images of 4T1 cells stained with FITC-2TT-oC6B nanoparticles and LysoTracker Red. Scale bar: 10 μm. b) Cell survival rates of 4T1 cells exposed to different concentrations of 2TT-oC6B nanoparticles, with or without NIR (near-infrared) and/or RT treatment. c) CLSM images of 4T1 cells exposed to different concentrations of 2TT-oC6B nanoparticles, with or without NIR and/or RT treatment. Scale bar: 100 μm. d) Qualitative representation of γ-H2AX foci formation. Scale bar: 10 μm. e) Photos of cell colony formation. (NPs: 2TT-oC6B nanoparticles; NIR: 808 nm, 1 W/cm2; RT: 4 Gy).
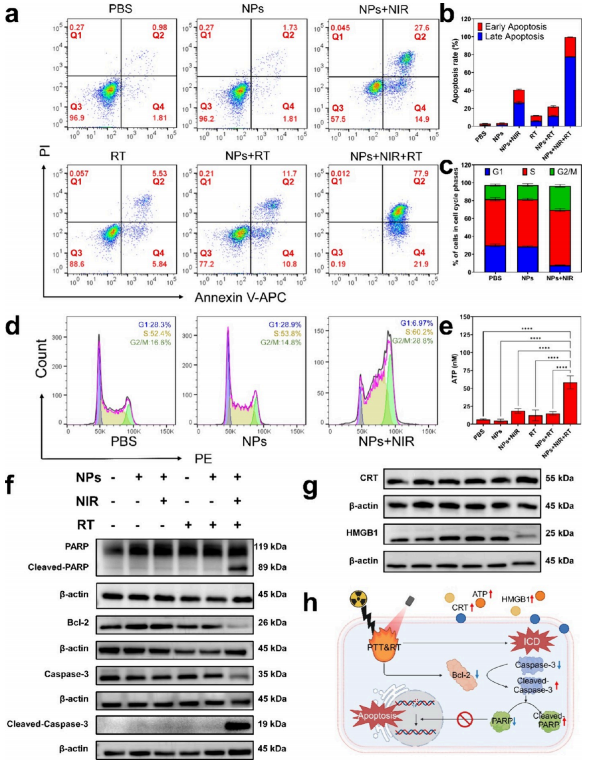
Figure 3. Efficacy and mechanisms of combining photothermal therapy (PTT) with radiotherapy (RT) at the cellular level. a) Apoptosis analysis of 4T1 cells treated differently by flow cytometry. Q1, percentage of necrotic cells (PI/APC, ±); Q2, percentage of late apoptotic cells (PI/APC, +/+); Q3, percentage of live cells (PI/APC,−/−); Q4, percentage of early apoptotic cells (PI/APC, ∓). b) Quantification of the percentage of apoptotic cells in panel (a). c) Quantification of the cell cycle in panel (d). d) Cell cycle analysis of 4T1 cells treated differently by flow cytometry. e) Quantification of extracellular ATP in 4T1 cells after different treatments. f) Western blot analysis of apoptosis-related proteins. g) Western blot analysis of immunogenic cell death (ICD) related proteins. h) Schematic diagram of the molecular mechanism of cell death induced by laser irradiation (created with BioRender.com). (NPs: 2TT-oC6B nanoparticles; NIR: 808 nm, 1 W/cm2; RT: 4 Gy).
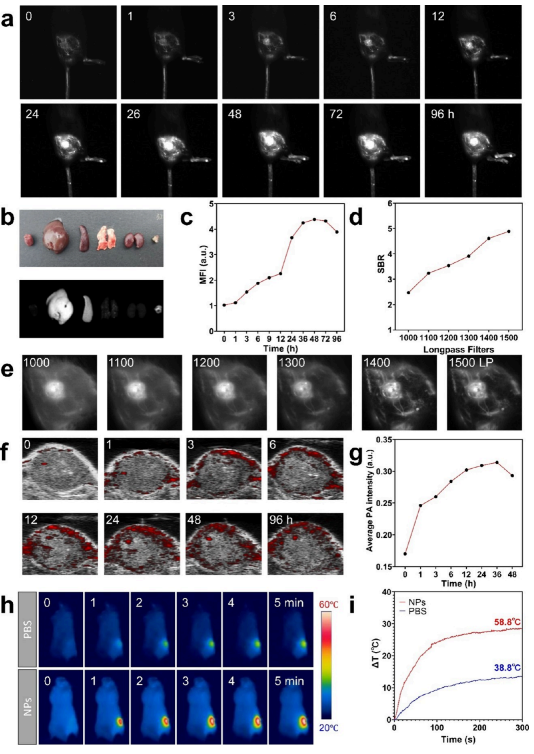
Figure 4. Multimodal imaging performance of 2TT-oC6B nanoparticles in BALB/c mice bearing 4T1 tumors. a) NIR-II fluorescence imaging (FLI) of tumor tissues at different monitoring time points after intravenous injection of 2TT-oC6B NPs (1 mg/mL, 200 μL). b) In vitro NIR-II FLI and bright field (BF) images of tumors and major organs 96 hours after intravenous injection of 2TT-oC6B NPs. c) Average NIR-II fluorescence intensity of tumor tissues at different monitoring time points corresponding to panel (a). d) Signal-background ratio changes under different LP filters at 9 hours, corresponding to panel (e). e) NIR-II FLI of tumor tissues using different LP filters at 9 hours. f) Photoacoustic imaging (PAI) of tumor tissues at different monitoring time points after intravenous injection of 2TT-oC6B NPs (1 mg/mL, 200 μL). g) Average PA intensity of tumor tissues at different monitoring time points corresponding to panel (f). h) Photothermal imaging (PTI) of tumor tissues under continuous laser irradiation (808 nm, 1 W/cm2, 0−5 minutes). i) Real-time temperature of tumor tissues in panel (h).
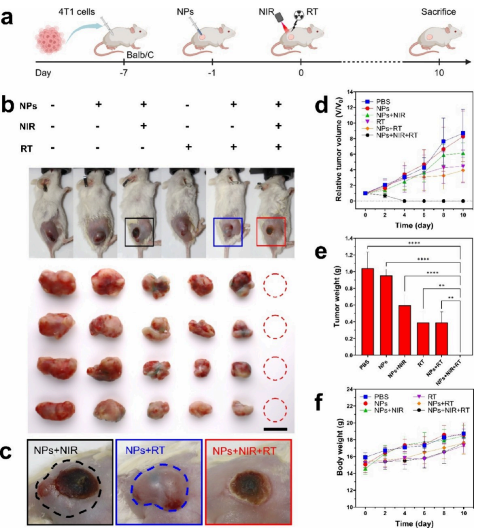
Figure 5. Antitumor effects of 2TT-oC6B nanoparticles in BALB/c mice bearing 4T1 tumors. a) Treatment schedule for combined photothermal therapy (PTT) and radiotherapy (RT) using 2TT-oC6B NPs. (Created with BioRender.com). b) Photos of mice and tumors at day 10 of different treatments. Scale bar: 10 mm. c) Photos of tumor areas after treatment with 2TT-oC6B NPs on day 10, with or without NIR and/or RT treatment (dashed line encircles tumor recurrence areas). d) Subcutaneous growth curves of 4T1 tumors in different treatment groups and e) weights. f) Weight changes of mice bearing subcutaneous 4T1 tumors in different treatment groups. (NIR: 808 nm, 1 W/cm2; RT: 4 Gy).
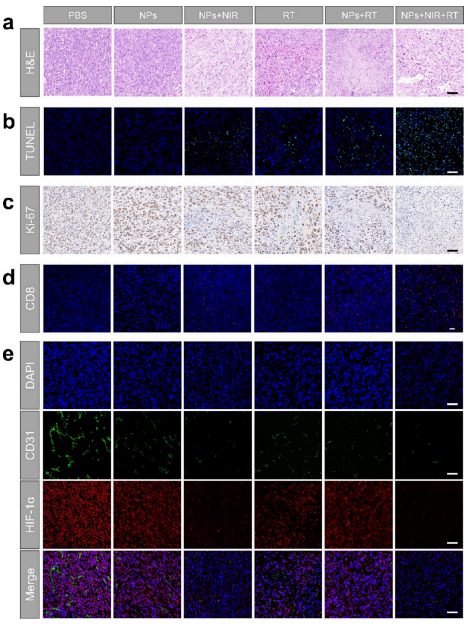
Figure 6. Efficacy and mechanisms of combining photothermal therapy (PTT) with radiotherapy (RT) at the tissue level in vivo. a-c) Hematoxylin and eosin (H&E), TUNEL, and Ki-67 staining analysis of tumor tissues after different treatments. Scale bar: 50 μm. d) Immunofluorescence images of infiltrating CD8+ T cells in tumor tissues. Scale bar: 50 μm. e) Immunofluorescence images of HIF-1α and CD31 in tumor tissues. Scale bar: 50 μm.

Journal: ACS NanoTitle: Organic Radiosensitizer with Aggregation-Induced Emission Characteristics for Tumor Ablation through Synergistic Apoptosis and Immunogenic Cell DeathAuthors: Dalu Xie, Xueke Yan, Wenzhao Shang, Hao Ren, Wei Wen, Ben Zhong Tang,* and Huifang Su*Acceptance Date: First published: 09 April 2025Original Link:: https://doi.org/10.1021/acsnano.5c00942Or click below to read the original text Or add the WeChat number below to invite you to join the group(Please specify: Name, School)
Or add the WeChat number below to invite you to join the group(Please specify: Name, School)
 Follow the application of luminescent materials and devices
Follow the application of luminescent materials and devices