Postoperative treatment of bone tumors faces numerous challenges, the most prominent of which is the high recurrence rate of tumors. This is closely related to the inability of implants to effectively reverse the polarization state of M2 macrophages and the loss of tumor phagocytosis caused by residual tumor cells. M2 macrophages play an immunosuppressive role in the tumor microenvironment, promoting tumor recurrence and metastasis. Therefore, developing implants that can regulate macrophage polarization and enhance their phagocytic function is of great significance in reducing tumor recurrence rates. 3D printing technology, due to its highly designable shapes and internal structures, as well as its portable therapeutic systems, shows great potential in the postoperative treatment of bone tumors. However, current 3D printed implants mainly focus on bone tissue regeneration and lack anti-tumor properties. Thus, this study aims to develop a multifunctional therapeutic implant that integrates CSF-1R inhibitors and anti-SIRPα antibodies through a co-delivery system to activate tumor-phagocytic macrophages, inhibit tumor recurrence, and promote bone regeneration, providing a new strategy for postoperative treatment of bone tumors.

Wang Jinwu and others from Shanghai Jiao Tong University developed a strategy to integrate macrophage regulatory functions into 3D printed calcium phosphate-based scaffolds. These implants not only effectively reduce postoperative tumor recurrence but also accelerate the repair of bone defects, improving patient survival rates and quality of life.
1. Main Content:
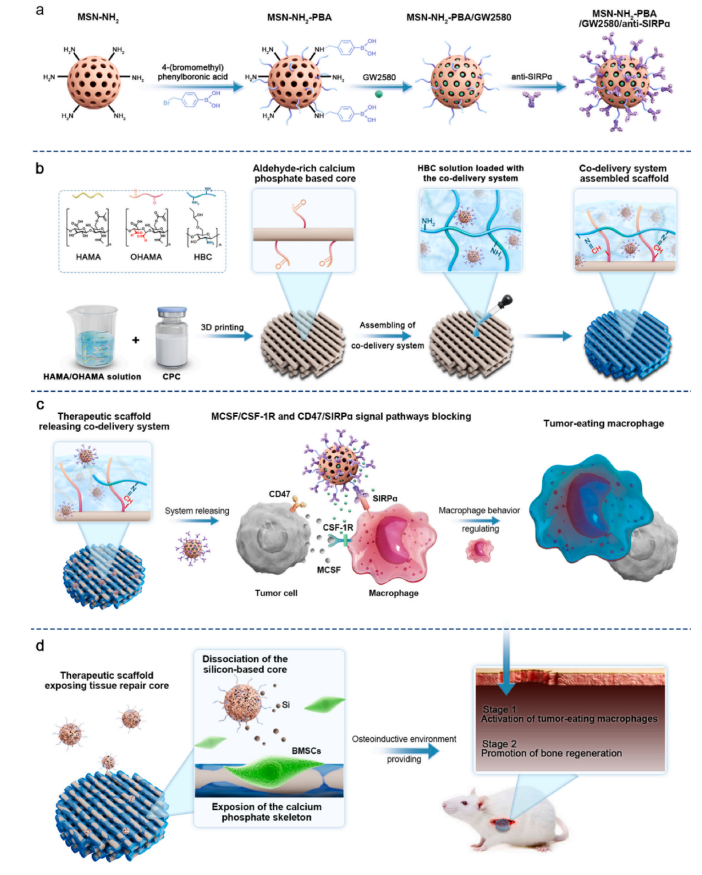
Figure 1 Design and Preparation of the Scaffold
The design of the scaffold is based on self-setting calcium phosphate cement (CPC), which can rapidly recrystallize into biodegradable nano-hydroxyapatite at room temperature through ion exchange when mixed with water, exhibiting excellent bone-guiding properties and plasticity. To enhance the mechanical properties and printability of the scaffold, the researchers added methacrylated hyaluronic acid (HAMA) and oxidized methacrylated hyaluronic acid (OHAMA) as photopolymerization setting solutions in the CPC. Using 3D printing technology, CPC was made into scaffolds with precise shapes and internal porous structures that facilitate bone tissue regeneration and nutrient transport.
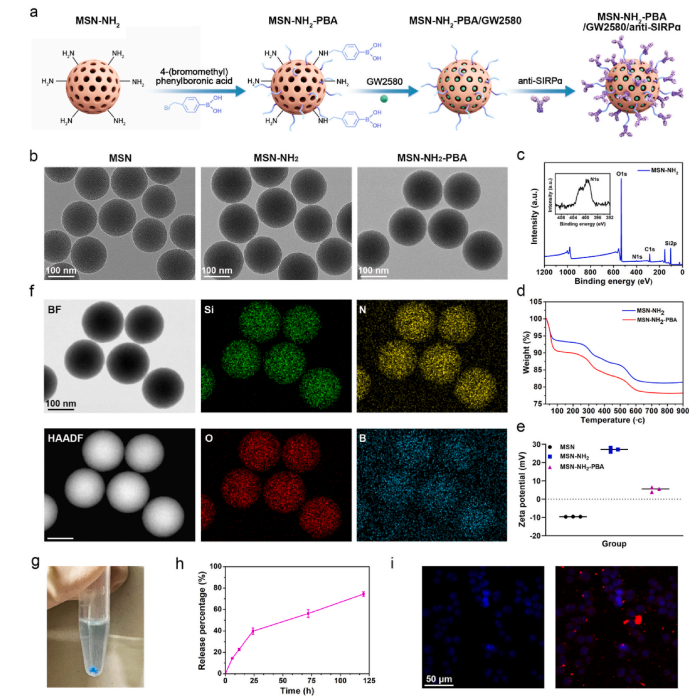
Figure 2 Preparation and Characterization of the Co-Delivery System
First, phenylboronic acid-modified mesoporous silica nanoparticles (MSN–NH2–PBA) were synthesized. These particles have efficient drug carrier functions, capable of enriching CSF-1R inhibitors and stably binding anti-SIRPα antibodies. Through dynamic covalent bonds, these nanoparticles were uniformly integrated onto the surface of the CPC scaffold, forming a sustained release layer.This design not only enables sustained drug release but also protects the core of bone repair from the influence of the tumor microenvironment.
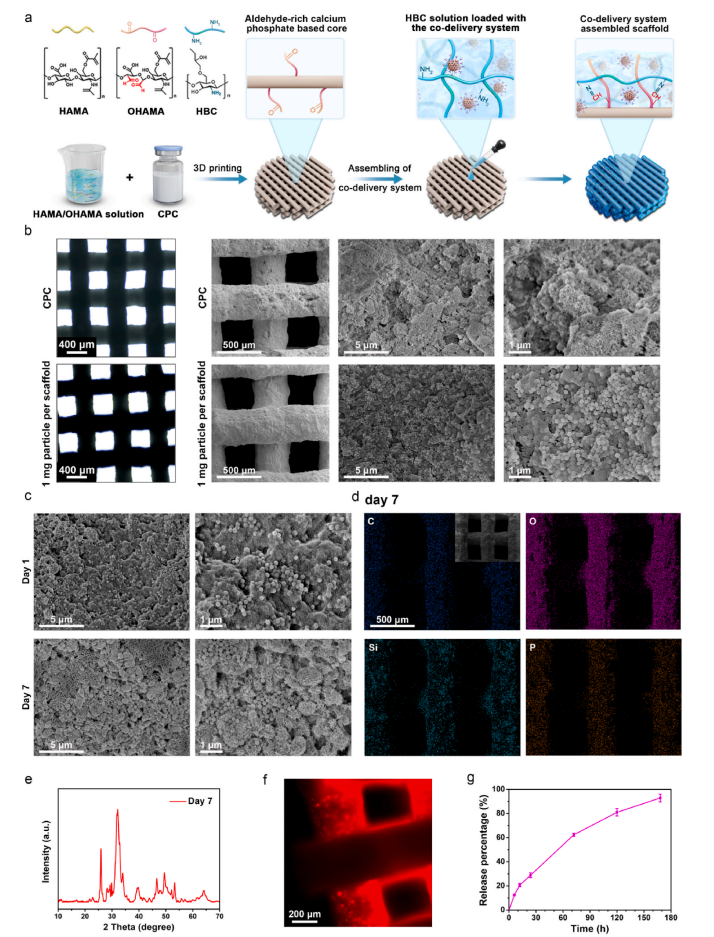
Figure 3 Preparation and Characterization of the Assembled Scaffold with Co-Delivery System
The scaffold has an aperture of approximately 400 micrometers, which aids in fluid exchange, nutrient transport, and bone tissue growth. Through scanning electron microscopy (SEM) and energy-dispersive spectroscopy (EDS) analysis, the researchers confirmed the uniform distribution and stability of the nanoparticles on the scaffold surface. Additionally, the surface morphology and elemental distribution of the scaffold after soaking in PBS for 7 days indicated that the scaffold has good stability and biodegradability.The scaffold was able to continuously release the inhibitor during the experiment without significant burst release phenomena. This sustained release characteristic is crucial for inhibiting tumor recurrence and regulating the immune microenvironment.
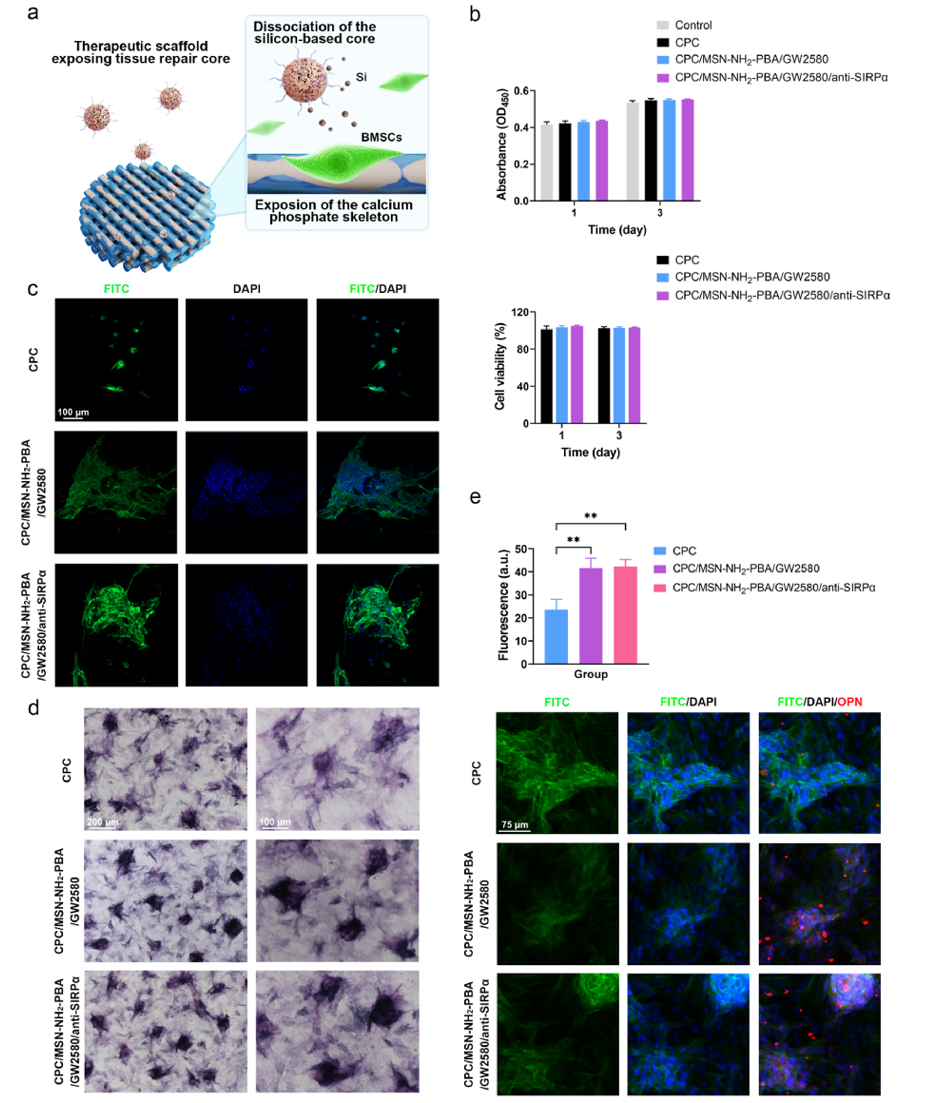
Figure 4 Effects of the Co-Delivery System on BMSCs Function
Further evaluation of the effects of the co-delivery system on the function of bone marrow mesenchymal stem cells (BMSCs) was conducted. Experimental results indicated that the released drugs did not have significant negative effects on the proliferation and osteogenic differentiation of BMSCs. On the contrary, drug-treated BMSCs exhibited stronger alkaline phosphatase (ALP) activity and higher osteopontin (OPN) expression, suggesting that the co-delivery system may have the potential to promote bone regeneration.These findings provide strong support for the application of the scaffold in postoperative treatment of bone tumors.
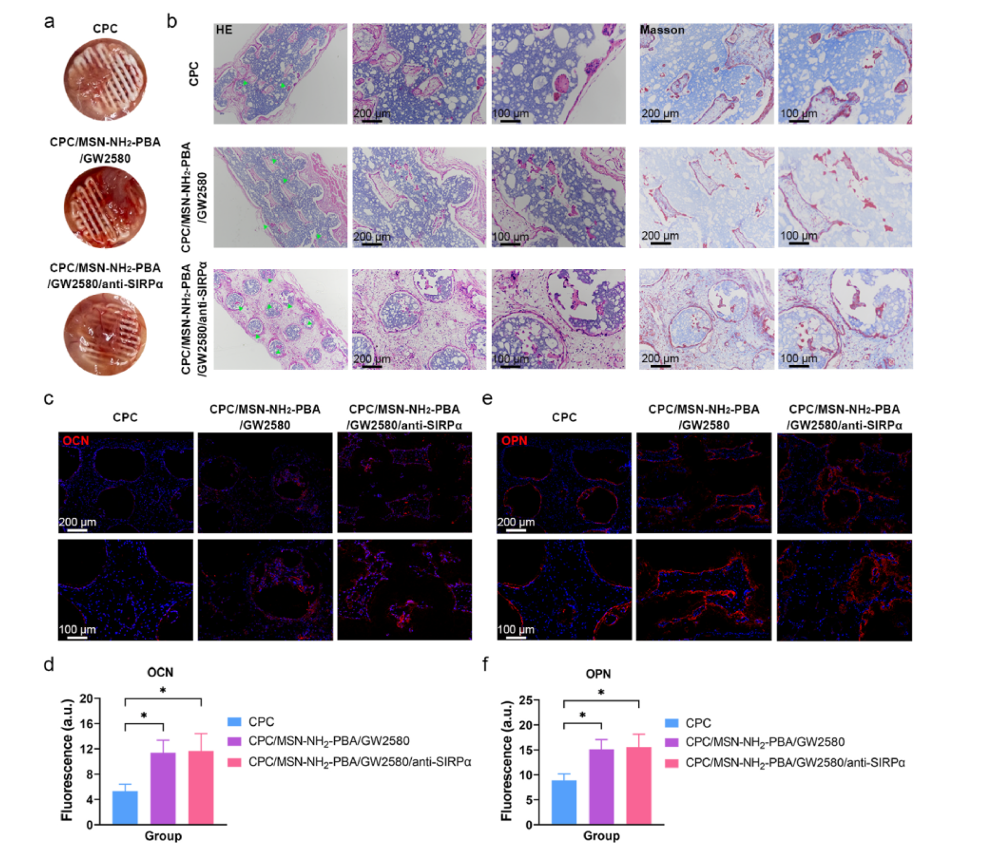
Figure 5 Biocompatibility and Bone Regeneration Ability of the Assembled Scaffold with Co-Delivery System
The biocompatibility and bone regeneration ability of the assembled scaffold with the co-delivery system were studied. In subcutaneous implantation experiments in normal mice, the scaffold exhibited good biocompatibility without causing significant inflammatory responses or tissue necrosis. Histological analysis showed that the scaffold could promote the formation of new bone tissue, primarily distributed around the printed filaments of the scaffold.Immunofluorescence staining results further confirmed the scaffold’s promoting effect on bone regeneration, indicating that the co-delivery system has potential application value in the postoperative treatment of bone tumors.
2. Summary of the Full Text:
This study developed an innovative 3D printed calcium phosphate scaffold that integrates CSF-1R inhibitors and anti-SIRPα antibodies through a co-delivery system, aiming to activate tumor-phagocytic macrophages and promote bone regeneration. The research team utilized phenylboronic acid-modified mesoporous silica nanoparticles (MSN-NH2-PBA) as drug carriers to stably bind CSF-1R inhibitor GW2580 and anti-SIRPα antibodies, assembling the co-delivery system onto the aldehyde-rich calcium phosphate scaffold through dynamic covalent bonds. Experimental results indicated that this system effectively blocks the signaling interactions between tumor cells and macrophages, inhibits M2 polarization of macrophages, maintains their phagocytic activity, suppresses tumor recurrence, and demonstrates good biocompatibility and ability to promote bone tissue repair in animal models.
References:
https://doi.org/10.1016/j.biomaterials.2025.123495
Source:EngineeringForLife
Disclaimer: The views expressed are solely those of the author and are for research purposes. The author’s level is limited; if there are any scientific inaccuracies, please leave comments below!
