Click the blue text to follow us! Abstract
Abstract
Developing antibacterial strategies based on reactive oxygen species (ROS) overcomes the extremely short diffusion distance of ROS and disrupts bacterial electron transfer, opening a promising yet underexplored avenue for non-antibiotic therapies. In this study, the authors introduced a bimetallic metal-organic framework (MOF) with peroxidase-like (POD) activity, designed to integrate two functions: bactericidal recognition and disruption of electron transfer to synergistically enhance antibacterial effects. Mechanistic studies indicate that the interaction between boronic acid and cis-diol allows the MOF to selectively bind to bacterial cell membranes, where it generates localized ROS to effectively kill bacteria. Meanwhile, the energy levels of the MOF match the redox potential of bacteria, facilitating efficient electron transfer from the bacterial cell membrane to the MOF, disrupting membrane integrity and inhibiting key processes such as electron transfer and ATP synthesis. When incorporated into biodegradable microneedle patches, this MOF can effectively penetrate biofilms and wound exudates, delivering powerful antibacterial effects directly to the site of infection while promoting tissue repair. This combination of bactericidal targeting, disruption of electron transfer, and microneedle-mediated delivery highlights the potential of this approach in advancing non-antibiotic antibacterial therapies. This research, titled“Bimetallic Metal−Organic Framework Microneedle Array for Wound Healing through Targeted Reactive Oxygen Species Generation and Electron Transfer Disruption,” was published inACS NANO.

 Background Introduction
Background Introduction
Bacterial infections remain a pressing global health challenge, with the overuse and misuse of antibiotics leading to an increase in resistant strains. Addressing bacterial resistance and the complex microenvironment of infected wounds requires innovative therapeutic approaches. Advances in the field of nanoenzymes present opportunities for non-antibiotic antibacterial strategies. Metal-organic frameworks (MOFs) with peroxidase-like (POD) catalytic activity have significant potential in antibacterial applications due to their large specific surface area, high porosity, and abundant metal active sites. Iron (Fe)-based MOFs can catalyze hydrogen peroxide into hydroxyl radicals through the Fe(III)/Fe(II) cycle, and integrating bimetallic structures, optimizing their design, and functionalizing them can enhance their catalytic efficiency and antibacterial performance.
However, the ROS-based antibacterial methods using MOF nanoenzymes face challenges, as the short lifespan of ROS limits their effective range and their lack of bacterial specificity in complex wound environments affects efficacy. Introducing boronic acid groups endows MOF nanoenzymes with targeting capabilities, which can overcome these issues.
Disrupting bacterial electron transfer is a complementary strategy to enhance bactericidal effects. Electron transfer is crucial for bacteria, and disrupting their electron transport chain can impair metabolic functions, leading to cell death. Self-antibacterial materials based on this principle have garnered attention, and MOFs can construct abiotic-biotic interfaces that interrupt bacterial electron transfer chains, but this mechanism has not yet been explored in MOF-based antibacterial therapies.
Moreover, effectively delivering therapeutic agents to the site of infection remains a critical challenge. Infected wounds are covered by biofilms, and wound exudates form barriers that hinder drug penetration, reducing the effectiveness of traditional antibacterial treatments, prolonging infection duration, and delaying wound healing.
 Research Overview
Research Overview
Microneedle patches consist of micrometer-scale needle arrays that enable efficient drug delivery. Unlike topical treatments that only act on the wound surface, they can penetrate biofilm layers and wound exudates, delivering therapeutic agents directly to the deeper tissues where bacteria reside. This method shortens the drug diffusion distance, reduces the risk of systemic side effects, ensures local drug concentration, and facilitates effective treatment. Inspired by these advantages, in this study, the authors developed a multifunctional microneedle system that integrates boronic acid-functionalized MOF as nanoenzymes for treating infected wounds (Scheme 1). Utilizing the key roles of iron and copper (Cu) in numerous natural enzymes, and the synergistic advantages of the bimetallic strategy, a simple method was employed to synthesize boronic acid-functionalized Fe/Cu bimetallic MOF (Fe/Cu – BBDC) at room temperature (Scheme 1A). Subsequently, the MOF nanoenzymes were incorporated into methacrylic acid hyaluronic acid (MAHA) microneedles to prepare MOF-based microneedle patches (MOF – MN, Scheme 1B). When applied to wounds infected with Staphylococcus aureus (S. aureus), the MOF – MN effectively delivered the MOF to the infected wound tissue, enhancing its antibacterial performance. The boronic acid groups specifically bind to the diol groups on the bacterial surface, further promoting the capture of the MOF. Additionally, the boronic acid-modified MOF forms abiotic/biotic interfaces upon contact with bacteria, and the energy levels of the MOF match the redox potential of bacteria, allowing electrons to be efficiently transferred from the bacterial cell membrane to the MOF, accelerating the bactericidal process. Furthermore, the weakly acidic environment and excess hydrogen peroxide present in infected wounds are utilized by the MOF to generate highly cytotoxic hydroxyl radicals (・OH), further enhancing antibacterial activity. This study highlights the excellent antibacterial performance and wound healing potential of MOF – MN, providing a promising approach for managing infected wounds and offering insights for the design of advanced self-antibacterial materials.

Scheme 1. Schematic diagram of the preparation of Fe/Cu-BBDC nanoenzyme-integrated microneedle patches (A and B) and their application in combating bacterial infections and promoting wound healing (C)
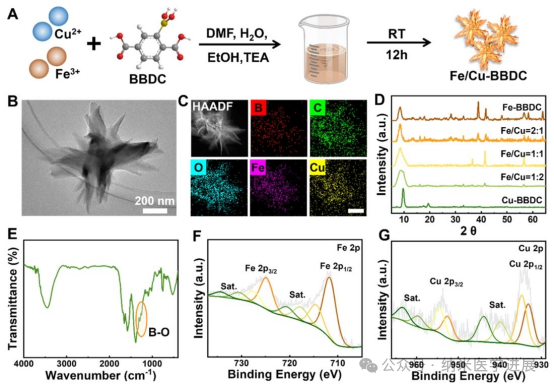
Figure 2. Preparation and characterization of Fe/Cu-BBDC metal-organic framework (MOF). (A) Preparation process of Fe/Cu-BBDC. (B) Transmission electron microscopy (TEM) images of Fe/Cu-BBDC (with molar ratios of Fe and Cu being 2:1, 1:1, and 1:2) and Cu-BBDC. (C) Scanning transmission electron microscopy (STEM) images and elemental mapping of Fe/Cu-BBDC. (D) X-ray diffraction (XRD) patterns of Fe-BBDC and Fe/Cu-BBDC (with different molar ratios of Fe and Cu). (E) Fourier-transform infrared spectroscopy (FTIR) of Fe/Cu-BBDC. (F) High-resolution X-ray photoelectron spectroscopy (XPS) of the Fe 2p orbitals in Fe/Cu-BBDC. (G) High-resolution XPS of the Cu 2p orbitals in Fe/Cu-BBDC.
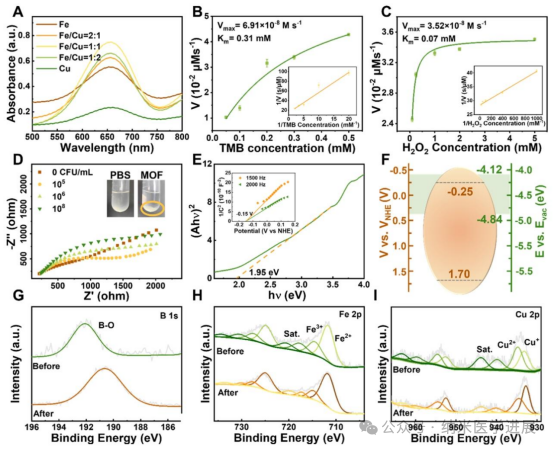
Figure 3. Peroxidase-like (POD) activity, bacterial capture ability, and bactericidal potential of Fe/Cu-BBDC. (A) UV-visible absorption spectra of oxidized 3,3′,5,5′-tetramethylbenzidine (oxTMB) in the presence of Fe-BBDC, Fe/Cu-BBDC (with molar ratios of Fe and Cu being 2:1, 1:1, and 1:2), and Cu-BBDC. (B) Michaelis-Menten kinetics curves of Fe/Cu-BBDC using TMB (C) and hydrogen peroxide (H2O2) as substrates. The inset shows the Lineweaver-Burk plot. (D) Nyquist plot of Fe/Cu-BBDC electrodes immersed in different concentrations of Staphylococcus aureus liquid cultures. The inset shows a photo of S. aureus aggregation induced by Fe/Cu-BBDC. (E) Bandgap of Fe/Cu-BBDC obtained from UV-visible diffuse reflectance spectroscopy. The inset shows the Mott-Schottky curve of Fe/Cu-BBDC. (F) Energy level diagram of Fe/Cu-BBDC, with the gray-green area indicating the range of biological redox potentials. (G) B 1s peak, (H) Fe 2p peak, and (I) Cu 2p peak before and after co-culturing Fe/Cu-BBDC with Staphylococcus aureus.
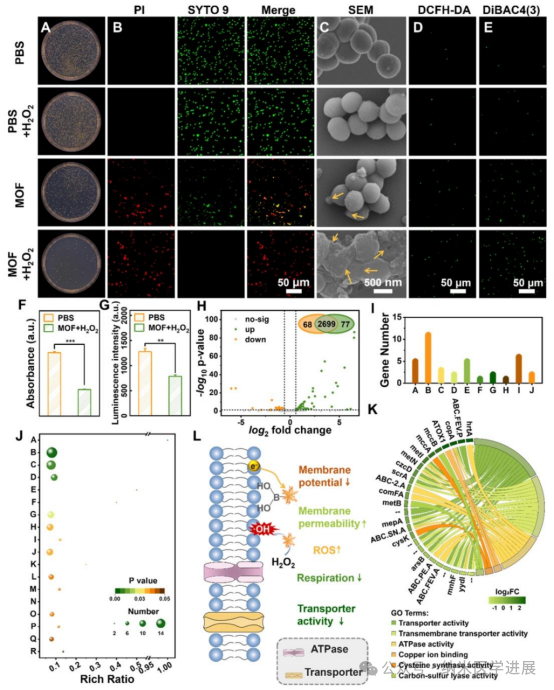
Figure 4. Antibacterial performance and mechanism study of Fe/Cu-BBDC. (A) Representative colony images of Staphylococcus aureus under different treatments, (B) live/dead staining images. Green fluorescence indicates live Staphylococcus aureus stained with SYTO 9, while red fluorescence indicates dead bacteria stained with propidium iodide (PI). (C) Scanning electron microscopy (SEM) images of Staphylococcus aureus morphology under different treatments. (D) Confocal laser scanning microscopy (CLSM) images detecting intracellular reactive oxygen species (ROS) in Staphylococcus aureus using 2,7-dichlorodihydrofluorescein diacetate (DCFH-DA) under different treatments. (E) Analysis of the membrane potential of Staphylococcus aureus using DiBAC4(3) staining. (F) Monitoring the respiration of Staphylococcus aureus using reduction-sensitive nitro blue tetrazolium (INT). (G) ATP expression levels of Staphylococcus aureus after 2 hours under dark conditions with different treatments. (H) Volcano plot of the MOF/$H_{2}O_{2}$ group compared to the control group, showing the number of genes. (I) Top ten signaling pathways ranked by Kyoto Encyclopedia of Genes and Genomes (KEGG). A: Cysteine and methionine metabolism; B: ABC transporters; C: Sulfur metabolism; D: Biotin metabolism; E: Quorum sensing; F: Carotenoid biosynthesis; G: Galactose metabolism; H: Ascorbate and aldarate metabolism; I: Amino acid biosynthesis; J: Phosphate transferase system (PTS). (J) Gene ontology (GO) pathways related to molecular functions. A: Cysteine synthase activity; B: Transporter activity; C: Transmembrane transporter activity; D: ATPase activity; E: Carbon-sulfur lyase activity; F: Copper ion binding; G: Nucleotide triphosphatase activity; H: Pyrophosphatase activity; I: Pyridoxal phosphate binding; J: Hydrolytic enzyme activity acting on anhydrides; K: Nickel cation binding; L: Active transmembrane transporter activity; M: Vitamin B6 binding; N: Methylenetetrahydrofolate reductase (NAD(P)H) activity; O: Ion transmembrane transporter activity; P: ATPase activity coupled to substance transmembrane movement; Q: Lyase activity; R: Transferase activity of alkyl or aryl groups. (K) Chord diagram obtained from GO pathway analysis. Unnamed genes are represented as “–“. (L) Antibacterial mechanism diagram of Fe/Cu-BBDC..
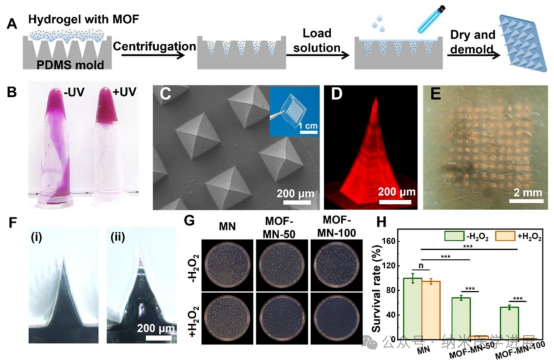
Figure 5. Preparation and characterization of Fe/Cu-BBDC-loaded microneedle (MOF-MN) patches. (A) Schematic diagram of the preparation process of MOF-MN patches. (B) Images of rhodamine B (Rh-B) modified methacrylic acid hyaluronic acid (MAHA) hydrogels before and after UV irradiation. (C) Scanning electron microscopy (SEM) images of MOF-MN patches. The inset shows optical images of the microneedles. (D) Fluorescence microscopy images of microneedles loaded with Rh-B. (E) Representative microscopic photos after inserting Rh-B loaded microneedles into pig skin. Each bright spot corresponds to the insertion point of each microneedle into the skin. (F) Morphological characteristics of MOF-MN before (i) and after (ii) incubation with simulated tissue fluid. (G) Representative colony images of Staphylococcus aureus under different treatments, (H) corresponding bacterial survival rates.
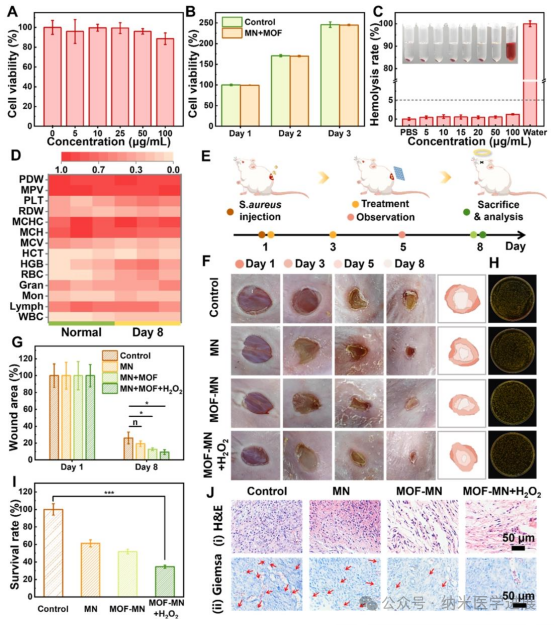
Figure 6. Biocompatibility and in vivo antibacterial performance. Cell toxicity of MOF (A) and MOF-MN (B) against HaCaT cells determined by MTT assay. (C) Hemolysis rate of red blood cells after incubation with different concentrations of MOF. The inset shows optical images. (D) Blood routine examination results of mice after MOF-MN treatment. (E) Schematic diagram of MOF-MN mediated treatment using Staphylococcus aureus infected wounds as a model. Photos of wounds after different treatments (F) and corresponding quantitative analysis (G). Photos of bacterial colonies from skin tissue obtained from wounds on day 8 (H) and corresponding quantitative analysis (I). Hematoxylin-eosin (H&E) stained images of wound tissue (i) and Giemsa stained images (ii).
 Conclusion and Discussion
Conclusion and Discussion
In summary, the authors introduced a boron capture strategy through a simple method, endowing bimetallic MOF nanoenzymes with recognition and antibacterial functions to enhance their antibacterial capabilities. Specifically, the prepared MOF serves as a targeted antibacterial agent capable of capturing bacteria and eradicating them through its POD-like activity and disruption of bacterial electron transfer. In vitro and in vivo experimental results indicate that the bimetallic MOF (Fe/Cu-BBDC) alters the membrane potential, membrane integrity, and oxidative stress state of bacteria, thereby affecting their respiration, ultimately promoting the healing of Staphylococcus aureus infected wounds through the use of MOF-loaded microneedles. Therefore, the MOF-MN patches can effectively treat bacterial infected wounds, providing a reference for developing antibacterial MOFs with targeted self-sterilizing functions.
For further clinical applications of this MOF-MN patch, issues such as the lack of personalized drug release, long-term safety, and insufficient skin adhesion are seen as critical problems that need to be addressed. As a comprehensive study of MOF-MN, developing smart diagnostic and therapeutic integrated patches with real-time monitoring capabilities is crucial for advancing the clinical translation of microneedle systems.
Paper link (DOI):
https://doi.org/10.1021/acsnano.5c02923
Disclaimer:This WeChat account forwards content solely for academic exchange purposes and does not serve commercial purposes, nor does it represent support or endorsement of its views. If there are issues related to intellectual property protection or other matters, please contact the editor in a timely manner, and we will coordinate to address them as soon as possible. The copyright of original articles from this WeChat account belongs to this WeChat account, and sharing on non-media platforms such as Moments is welcome.
Contact the editor for free submissions. This public account also has a real-name academic exchange group for nanomedicine, please note your name + institution + research direction to add the WeChat account below.
Previous recommendations: Professor Huang Haishui from Xi’an Jiaotong University in Bioactive Materials: Cell-loaded patches for the treatment of diabetic ulcers that can be cryopreserved, scalable, and ready-to-use
Click “Read the original text” to access the original paper
「ENDING」


Submission WeChat|nanomedicine1
Submission Email|[email protected]
Follow the progress of nanomedicine
Learn about the forefront of nanomedicine
Long press the QR code to submit privately