
Abstract: Intrahepatic cholangiocarcinoma (ICC) is a relatively rare type of primary liver cancer, with an increasing incidence in recent years. Due to its insidious onset and atypical clinical symptoms, most patients are diagnosed at an advanced stage of the disease, making timely diagnosis and treatment crucial. The standard treatment for early-stage ICC patients is radical surgical resection, while systemic chemotherapy is the basis for treatment in advanced-stage patients, combined with interventional therapy, targeted therapy, and immunotherapy. This article aims to review the advances in the diagnosis and treatment of ICC.
Keywords: Cholangiocarcinoma; Diagnosis; Treatment

Intrahepatic cholangiocarcinoma (ICC) originates from the epithelial cells of the intrahepatic bile ducts and is a type of bile duct carcinoma that occurs above the secondary bile ducts in the liver. The incidence of ICC is second only to hepatocellular carcinoma (HCC), accounting for 10% to 15% of primary liver cancers and approximately 20% of bile duct cancers. The incidence of ICC has been rising globally, with a 140% increase over the past 40 years. ICC is a highly malignant tumor, and despite various treatment options such as surgery, chemotherapy, radiotherapy, interventional therapy, targeted therapy, and immunotherapy, the mortality rate of ICC has not decreased worldwide, making it one of the significant diseases threatening human health.
1Risk Factors for ICC
ICC typically develops in the context of chronic inflammation, which leads to bile stasis and damage to bile duct cells. The occurrence of ICC is associated with various risk factors, including advanced age, bile duct stones, bile duct adenomas, bile duct papillomatosis, Caroli disease, choledochal cysts, viral hepatitis, liver cirrhosis, primary sclerosing cholangitis, ulcerative colitis, chemical toxins, and smoking. Metabolic syndrome (including obesity, diabetes, and non-alcoholic fatty liver disease) and excessive alcohol consumption are also closely related to the occurrence of biliary tumors. Additionally, the occurrence of ICC is associated with genetic variations, such as DNA repair, inflammation, metabolism of carcinogens, and stress after gene defects.
2Staging of ICC
The most commonly used staging system for ICC is the tumor-node-metastasis (TNM) staging. The eighth edition of the cancer staging manual has revised prognostic factors such as tumor size, T staging, and lymph node positivity. The main content includes: (1) T1 tumors are divided into T1a (tumor length ≤5cm) and T1b (tumor length >5cm); (2) T2a and T2b are combined into T2 due to similar prognostic value regarding intrahepatic vascular invasion and multiple lesions; (3) T4 has been updated to indicate direct invasion of the tumor into extrahepatic tissues due to the controversial prognostic value of tumor growth patterns in ICC.
3Diagnosis of ICC
Early ICC lacks specific clinical symptoms and may only present with slight changes in liver function, often being incidentally discovered as an isolated intrahepatic mass during imaging examinations. As the disease progresses, patients may experience abdominal pain, fatigue, nausea, upper abdominal masses, fever, and jaundice. ICC patients lack specific clinical manifestations, and for those with a high suspicion of ICC, diagnosis requires a comprehensive assessment of clinical symptoms, laboratory tests, imaging studies, and pathological examinations (Figure 1).
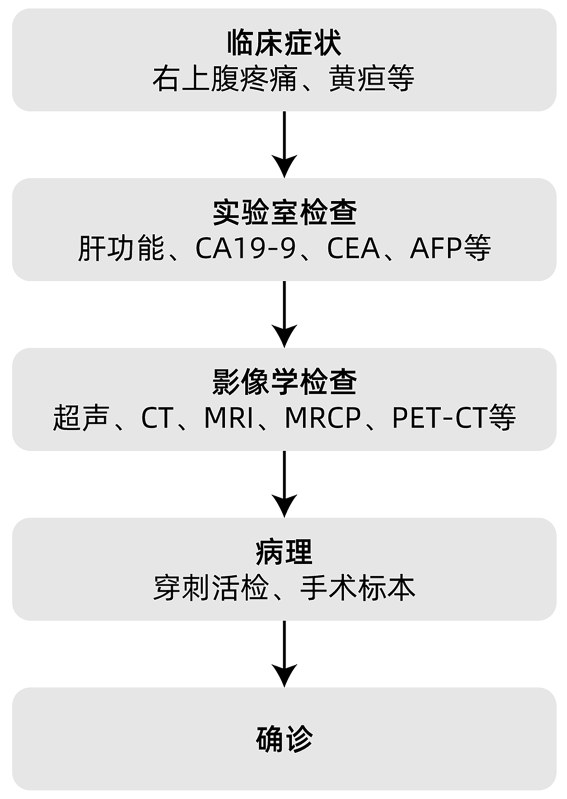
Note: CA19-9, carbohydrate antigen 19-9; CEA, carcinoembryonic antigen;
Figure 1 ICC Diagnosis Flowchart
3.1 Laboratory Tests Liver function tests can provide preliminary diagnostic references for ICC, but their specificity is low. CA19-9 and CEA are the most commonly used serum markers for diagnosing ICC, although their specificity is also not ideal, they have value in auxiliary diagnosis and predicting treatment efficacy. Most ICC patients have elevated levels of CA19-9 and CEA, and the degree of elevation is associated with tumor metastasis and poor prognosis. In recent years, with the development of molecular biology, small RNAs, free DNA, circulating tumor cells, cytokeratin (CK) 19 fragments, and serum extracellular vesicles in blood or bile have become new laboratory test indicators. Therefore, for suspected ICC patients, screening for CA19-9, CEA, AFP, and other indicators should be conducted, and hospitals with conditions can perform molecular biological tests.
3.2 Imaging Studies Due to the lack of specific clinical manifestations and laboratory test results for ICC, imaging studies have become the first choice. They can not only screen high-risk populations and provide diagnostic evidence but also determine treatment plans and assess efficacy. Common examination methods include ultrasound, CT, MRI, and PET-CT.
Ultrasound is convenient and non-invasive, making it the preferred imaging examination method for suspected ICC patients. However, ultrasound is difficult to differentiate ICC from other types of space-occupying lesions and to accurately assess the resectability of the tumor.
CT and MRI are important examination methods for ICC, accurately assessing the tumor’s location, size, and relationship with surrounding tissues. CT is superior to MRI in assessing the resectability of ICC. MRI has higher tissue resolution and is more advantageous than CT in showing lesions and the extent of surrounding liver tissue involvement. MRCP can visually display the location of ICC, differentiate it from extrahepatic bile duct tumors, and design surgical plans by analyzing the anatomical relationship between the tumor and the bile duct system.
PET-CT can detect ICC with a diameter of <1cm, showing excellent results in detecting distant metastasis and lymph node infiltration, superior to CT and MRI. However, the high cost and low spatial resolution limit its clinical application.
3.3 Pathological Diagnosis and Molecular Typing Biopsy remains the gold standard for diagnosing ICC. The pathology of ICC often presents as tubular or papillary adenocarcinoma with some variations in fibrous stroma. Due to the presence of more fibrous tissue and fewer blood vessels, ICC tissue appears whiter and harder than HCC tissue, with immunohistochemistry showing CK7 and CK20 positivity.
In recent years, molecular typing based on gene expression profiles and key gene mutations has become a research hotspot. The comprehensive application of epigenetics, genomics, and proteomics for molecular typing of ICC has significant clinical implications. Some researchers have classified ICC into inflammatory and proliferative types based on integrated genomic and transcriptomic features, with the former characterized by activation of inflammatory signaling pathways, overexpression of cytokines, and activation of STAT3; the latter characterized by activation of oncogenic signaling pathways (RAS/MAPK, MET, EGFR, ERBB2, NOTCH, etc.) related to cell proliferation, indicating a higher degree of malignancy. Inflammatory-type ICC is associated with KRAS mutations, TP53 mutations, GNAS mutations, and high COX2 expression, while IDH gene mutations are almost absent; IDH1/2 and BRAF mutations, as well as FGFR2 gene fusions, occur only in small bile duct-type ICC. Therefore, molecular pathological examination for typing ICC can help understand the presence of targeted genes in tumor cells, guiding the selection of clinical targeted drug therapies.
Dong et al. conducted a systematic molecular analysis of ICC centered on proteomics, establishing a molecular typing based on proteogenomics, including four subtypes: inflammatory (S1), stromal (S2), metabolic (S3), and differentiated (S4). These four subtypes have unique characteristics in genomics, immune microenvironment, treatment strategies, and clinical prognosis, which are expected to guide individualized clinical treatment. Through dimensionality reduction analysis, markers that can specifically distinguish the four subtypes were identified, confirming their potential for clinical sample typing, with HKDC1 and SLC16A3 identified as prognostic biomarkers for ICC. It is believed that with further research into the pathogenesis of ICC and continuous improvement of molecular typing, more favorable conditions for the development of precise treatment for ICC will be established.
4Treatment of ICC
4.1 Treatment of Early ICC For early-stage ICC patients, the standard treatment is radical surgical resection, but only about 35% of patients can be diagnosed early, with a 5-year survival rate of about 30%, and a postoperative 5-year recurrence rate as high as 60% to 70%. Therefore, postoperative adjuvant therapy for ICC has received much attention. Some studies have shown that postoperative adjuvant therapy is an independent factor affecting the survival of cholangiocarcinoma patients. The BILCAP phase III trial included 447 patients with cholangiocarcinoma or gallbladder cancer who underwent radical resection, randomly assigned to receive adjuvant capecitabine or no adjuvant treatment. The results showed that the overall survival (OS) in the capecitabine group was 51.1 months, compared to 36.4 months in the observation group (P=0.028). Based on these trial results, guidelines recommend 6 months of capecitabine adjuvant therapy as the standard adjuvant treatment after ICC resection.
Neoadjuvant therapy is aimed at initially resectable ICC, with the goal of shrinking the lesions and improving the R0 resection rate. Currently, there is a lack of prospective research evidence showing that neoadjuvant therapy benefits the prognosis of ICC. A retrospective study indicated that neoadjuvant chemotherapy could control hidden metastases and reduce the risk of recurrence. Other studies have suggested that neoadjuvant chemotherapy tends to extend survival time compared to surgery alone, but the differences in final results are not statistically significant.
4.2 Treatment of Advanced ICC
4.2.1 Interventional Therapy For patients with unresectable ICC, local interventional therapies can be employed, including transcatheter arterial chemoembolization (TACE), transarterial radioembolization (TARE), hepatic arterial infusion chemotherapy (HAIC), and ablation. So far, TACE and TARE have not shown satisfactory treatment effects in patients with unresectable ICC, possibly because ICC is a tumor with poor blood supply, thus more inclined to combined radiochemotherapy or targeted and immunotherapy. Interventional therapy may modulate the tumor microenvironment by exposing tumor antigens, increasing immunogenicity, reducing tumor burden, and promoting inflammatory environments, thereby enhancing responses to immunotherapy. Studies have shown that interventional therapy combined with chemotherapy can extend patient survival and provide survival benefits. Large randomized controlled trials of TACE combined with targeted and/or immune checkpoint inhibitors in ICC have been lacking, and multiple combination treatment trials are currently underway (NCT05247996, NCT04954781, NCT05448183, and NCT05738057). In HCC, HAIC combined with lenvatinib and PD-1 inhibitors has shown significantly better efficacy compared to lenvatinib or PD-1 inhibitors alone; in malignant biliary tumors, lenvatinib combined with PD-1 inhibitors has also shown effectiveness. Therefore, the author looks forward to the efficacy and safety of HAIC combined with targeted and/or immunotherapy in ICC, with relevant clinical studies (NCT05400902, NCT05290116, NCT0534881) ongoing. Additionally, studies on the combination of cryoablation with immunotherapy and targeted therapy are also in progress (NCT05010668 and NCT04299581), with expectations for the efficacy and safety of combination treatments.
4.2.2 Systemic Chemotherapy Systemic chemotherapy is an important method for treating unresectable or metastatic ICC. The ABC-02 phase III randomized controlled trial showed that the median overall survival (OS) for the gemcitabine plus cisplatin (GC regimen) group was 11.7 months, compared to 8.1 months for the gemcitabine monotherapy group, with median progression-free survival (PFS) of 8 months and 5 months, respectively, with statistically significant differences. Based on these research results, guidelines recommend gemcitabine combined with platinum drugs as first-line chemotherapy for advanced ICC. Two other phase III studies have confirmed that gemcitabine combined with tegafur (GS regimen) and capecitabine combined with oxaliplatin (XELOX regimen) have efficacy not inferior to the GC regimen. Therefore, gemcitabine-based chemotherapy regimens can serve as the basic regimen for conversion therapy in patients with unresectable ICC.
In addition to dual-drug combination therapy, triple-drug combination therapy is also gradually becoming an important exploration direction for systemic treatment. Shroff et al. reported that the GC regimen combined with albumin-bound paclitaxel showed good efficacy for advanced cholangiocarcinoma. The KHBO1401 phase III randomized controlled study verified that the GC regimen combined with tegafur was superior to the GC regimen in unresectable or recurrent malignant biliary tumors. Furthermore, gemcitabine combined with albumin-bound paclitaxel and fluorouracil combined with platinum are also important systemic chemotherapy regimens for conversion therapy in ICC.
There are relatively few phase III large-sample clinical studies on second-line chemotherapy. The ABC-06 phase III clinical study showed that compared to supportive treatment alone, symptom control combined with the FOLFOX regimen (oxaliplatin + leucovorin + fluorouracil) as second-line treatment can provide survival improvement for patients with advanced biliary malignancies who progressed after first-line GC treatment, significantly extending the median OS. Based on these results, the FOLFOX regimen has become the standard second-line treatment for advanced cholangiocarcinoma.
4.2.3 Targeted Therapy With the development of molecular typing and gene testing technologies for ICC, targeted therapy for ICC can achieve precision treatment, with relatively mature treatment strategies targeting FGFR2 and IDH1.
FGFR fusions are common mutations, occurring in 10% to 15% of ICC cases. FGFR small molecule inhibitors are generally divided into two categories: pan/highly selective FGFR inhibitors and multi-targeted tyrosine kinase inhibitors. Currently, two FGFR inhibitors have been approved for the treatment of malignant biliary tumors, namely pemigatinib and infigratinib. The FIGHT-202 trial evaluated the efficacy of pemigatinib in patients with advanced malignant biliary tumors who had received prior treatment, showing an objective response rate (ORR) of 37.0% and a disease control rate (DCR) of 82.4% in the FGFR2 fusion or rearrangement population, with median PFS and OS of 7.0 months and 17.5 months, respectively. A bridging study conducted in China included 34 cholangiocarcinoma patients, showing an ORR of 56.7% and a PFS of 9.6 months for pemigatinib in the Chinese population. Based on these research results, in 2022, the National Medical Products Administration approved pemigatinib as the first FGFR inhibitor for second-line treatment in China, marking a breakthrough in targeted therapy for advanced cholangiocarcinoma.
IDH mutations are detected in approximately 25% of ICC cases. Ivosidenib is a small molecule inhibitor targeting IDH-1 mutant protein, which can maintain long-term disease stability in ICC patients and has good safety. It was approved by the U.S. Food and Drug Administration in 2021 for second-line treatment of locally advanced or metastatic cholangiocarcinoma in adults with IDH1 mutations who have received prior treatment. The results of the large phase III randomized controlled trial ClarIDHy showed that the median PFS and OS in the placebo group were 1.4 months and 5.1 months, respectively, while in the ivosidenib group, they were 2.7 months and 10.3 months, with 50.8% of patients achieving disease stability.
NTRK fusion positivity was found in 3.5% of ICC patients, and NTRK inhibitors entrectinib and larotrectinib are recommended, both showing certain efficacy. The ORR for entrectinib in patients with NTRK fusion-positive advanced tumors who had received prior systemic treatment reached 57%, with a median duration of response of 10 months and a median PFS of 11.2 months, while larotrectinib achieved an ORR of 75% in a phase II clinical trial in a similar population.
HER2 amplification and mutations account for 5% to 15% of patients with malignant biliary tumors, approximately 5% in ICC. A multicenter, single-arm phase II clinical trial (HERB trial) of trastuzumab for HER2-expressing unresectable or recurrent biliary malignancies showed certain efficacy, but its long-term efficacy and safety still need further exploration.
4.2.4 Immunotherapy Immune checkpoint inhibitors are currently the most commonly used immunotherapy drugs in cancer treatment, and extensive exploration has been conducted in cholangiocarcinoma treatment in recent years, with encouraging preliminary data. PD-1 antibody monotherapy can achieve good and durable efficacy in biliary cancer patients with high microsatellite instability (MSI-H), deficient mismatch repair (dMMR), and high tumor mutational burden (TMB-H). The KEYNOTE-158 study showed an ORR of 40.9%, with median PFS and OS of 4.2 months and 24.3 months, respectively; nivolumab also showed similar effects. Currently, the NCCN guidelines recommend pembrolizumab as a second-line and above treatment for advanced biliary cancer patients with MSI-H/dMMR. Compared to monotherapy, the combination of PD-1 and CTLA-4 inhibitors has shown superior performance in advanced solid tumors such as melanoma and colorectal cancer. A multicenter phase I study showed that advanced biliary cancer patients could tolerate the combination of PD-1 and CTLA-4 inhibitors and achieve better clinical benefits, with DCRs of 16.7% and 32.2% at 12 weeks for the monotherapy group (Group D) and the combination group (D+T), respectively, and OS of 8.1 months and 10.1 months, with statistically significant differences. The advantages of dual immune therapy are highlighted, but further large-sample, high-quality, prospective randomized controlled trials are needed to clarify their therapeutic effects and safety.
4.2.5 Combination Therapy There is limited research on the combination of immunotherapy and targeted drug therapy. A single-arm, open-label, phase II clinical study conducted by Professor Yan’s team demonstrated that PD-1 inhibitors combined with lenvatinib for treating unresectable advanced biliary cancer achieved an ORR of 42.1%, DCR of 76.3%, median PFS of 8 months, and median OS of 17.7 months, with 34.2% successfully achieving downstaging and conversion resection. This study provides a new treatment idea for conversion therapy in unresectable biliary cancer. The LEAP-005 study presented at the American Society of Clinical Oncology Gastrointestinal Cancers Symposium in 2021 indicated that pembrolizumab combined with lenvatinib has certain efficacy in advanced malignant biliary tumors. So far, there is no data supporting the combined application of immunotherapy with targeted drugs such as FGFR inhibitors, IDH1 inhibitors, or HER2-directed drugs in biliary tumors.
Regarding the combination of immunotherapy with systemic chemotherapy, the author believes this is a key focus for future development. The TOPAZ-1 trial evaluated the efficacy of the GC regimen combined with durvalumab compared to the GC regimen plus placebo, showing median OS of 12.8 months and 11.5 months, respectively, and median PFS of 7.2 months and 5.7 months, with statistically significant differences. The combination group reduced the risk of death by 20% in patients with unresectable advanced or metastatic biliary malignancies compared to chemotherapy alone. In terms of safety, there was no significant difference in grade 3-4 treatment-related adverse events between the combination and chemotherapy alone. These research results mark a new breakthrough in the treatment of advanced ICC, with guidelines recommending the combination of gemcitabine and platinum drugs, and where conditions permit, durvalumab can be added to this regimen. Based on the TOPAZ-1 study, the National Medical Products Administration approved durvalumab combined with the GC regimen for first-line treatment of advanced biliary cancer, confirming a new breakthrough in immunotherapy combination strategies in the biliary cancer field. The KEYNOTE-966 study is a randomized, double-blind, placebo-controlled international multicenter phase III clinical study aimed at evaluating the efficacy and safety of pembrolizumab combined with the GC regimen compared to placebo combined with the GC regimen in first-line treatment of advanced or unresectable cholangiocarcinoma patients, with a median OS of 12.7 months in the pembrolizumab group and 10.9 months in the control group (P=0.0034); 25% of patients in the pembrolizumab group were still alive at 24 months (18% in the control group). The results of the TOPAZ-1 and KEYNOTE-966 studies corroborate each other, and KEYNOTE-966 further confirms that the PD-1 inhibitor plus the GC regimen is expected to become the standard first-line treatment for advanced cholangiocarcinoma.
There is limited research on the combination of immunotherapy with targeted drugs and systemic chemotherapy. A single-arm, open-label phase II clinical trial of the “four-drug three-combination regimen” of toripalimab, lenvatinib, and gemcitabine plus oxaliplatin (GEMOX) for treating unresectable advanced ICC showed an ORR of 80% and an OS of 22.5 months, and the phase III clinical trial of this study has been approved. A multicenter, single-arm, retrospective study conducted by Professor Cheng’s team in 2023 explored the efficacy and safety of atezolizumab combined with bevacizumab and GEMOX for advanced biliary tumors, showing an ORR of 76.7% for the combination therapy, with an ORR of 73.3% for ICC and a PFS of 12.0 months.
Regarding new immunotherapies for ICC, there are tumor vaccines such as peptide vaccines, Wilm’s tumor protein 1 vaccine, and mucin 1 vaccine, as well as immune cell therapies such as chimeric antigen receptor T lymphocyte therapy and tumor-infiltrating lymphocyte therapy, which have made some preliminary clinical trial progress. However, the sample sizes of the trials are very limited, and more large-sample randomized controlled clinical studies are needed to verify their efficacy and safety.
5Conclusion and Outlook
The etiology of ICC is complex, with high invasiveness and an increasing incidence year by year. The early symptoms of this disease are not obvious, and most patients are already in the late stage of the tumor when symptoms appear. With the continuous development of diagnostic technologies, the early diagnosis rate of ICC is gradually improving. Multidisciplinary comprehensive diagnosis and treatment of ICC is particularly important, with the key being to optimize the combination of various treatment methods, fully reflecting the advantages of multidisciplinary teams (Figure 2). For early, resectable ICC patients, radical surgical resection is the main treatment, followed by adjuvant chemotherapy; for advanced, unresectable ICC patients, systemic chemotherapy is the standard treatment and the basis for combination therapy. The combined application of systemic chemotherapy, radiotherapy, interventional therapy, targeted therapy, and immunotherapy for ICC will be the direction of future exploration.
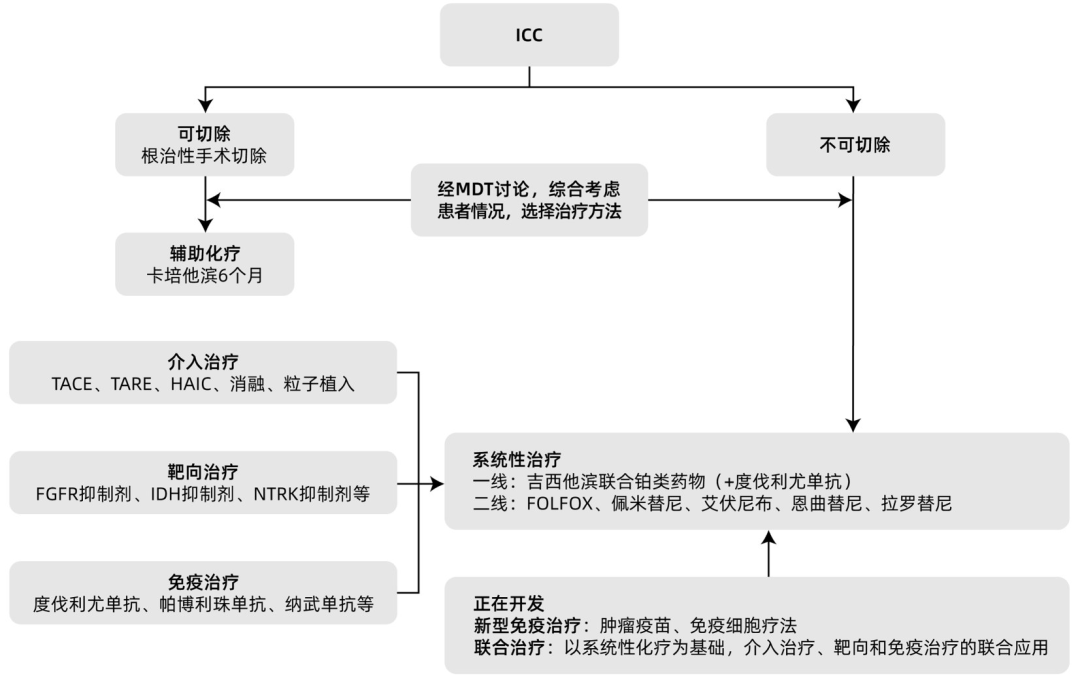
Figure 2 Treatment of ICC
 Citation
Citation
Hu Di,Huang Jintao,Zhong Binyan,et al..Advances in Diagnosis and Treatment of Intrahepatic Cholangiocarcinoma[J]. Clinical Journal of Hepatobiliary Diseases, 2024, 40(7): 1470-1476.