Introduction
The disproportionation reaction of rosin is a key step in the preparation of high-value-added chemical products. However, traditional catalysts face bottlenecks such as high costs and significant pollution. The team from Qingdao University of Science and Technology has developed a new Pd/C-N@SiO₂ catalyst that achieves efficient and green catalysis through hollow structure design and nitrogen doping technology, outperforming commercially available Pd/C catalysts!
“Click the video to understand the secrets of hollow structure catalysts in 3 minutes!”
👉 Read the original text: Get the full paper

Huang Zhenzhong, Xie Congxia, Yu Fengli, et al. Preparation, characterization and catalytic performance of Pd/C-N@ SiO2 catalyst for disproportionation reaction of rosin[J]. Chemistry and Industry of Forest Products, 2025, 45(1): 107-114.
HUANG Z Z, XIE C X, YU F L, et al. Preparation, characterization and catalytic performance of Pd/C-N@ SiO2 catalyst for disproportionation reaction of rosin[J]. Chemistry and Industry of Forest Products, 2025, 45(1): 107-114.
1 Experiment
1.1 Raw Materials, Reagents, and Instruments
1.2 Preparation of Pd/C-N@SiO2 Catalyst
1.2.1 Preparation of C-N@SiO2 Nanomaterials
1.2.2 Preparation of Pd/C-N@SiO2 Catalyst
1.3 Analysis and Characterization of the Catalyst
1.3.1 TEM Analysis
1.3.2 SEM Analysis
1.3.3 N2 Adsorption-Desorption Analysis
1.3.4 XRD Analysis
1.3.5 XPS Analysis
1.4 Preparation of Dehydroabietic Acid
1.5 Quantitative Analysis of Products
1.5.1 Product Pretreatment
1.5.2 GC Analysis
2 Results and Discussion
2.1 Analysis and Characterization of the Catalyst
2.1.1 SEM and TEM Analysis
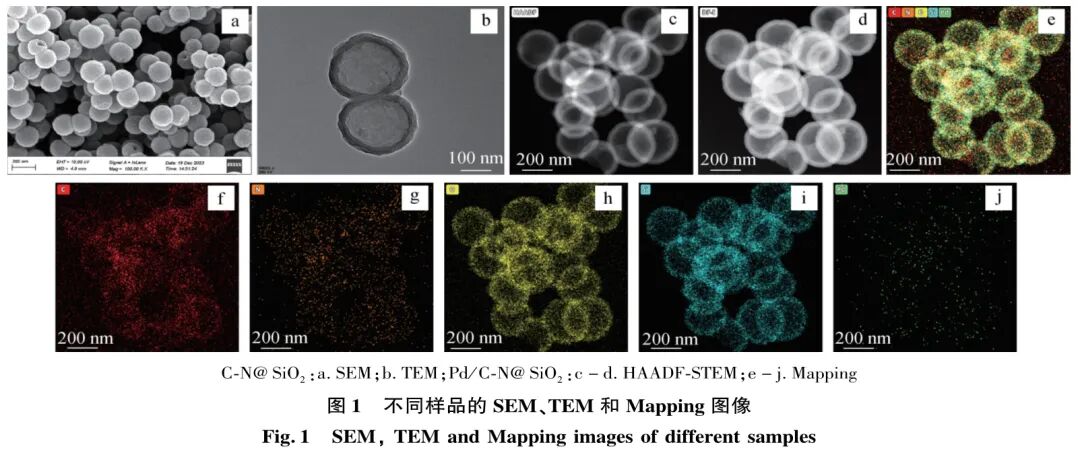
To prove that the material has a hollow nanostructure, SEM and TEM analyses were performed, as shown in Figure 1(a) and Figure 1(b). From Figure 1(a), it can be seen that the average particle size of C-N@SiO2 nanomaterials is 200 nm, and they exhibit good dispersion; the broken material images show a clear cavity structure. The TEM image in Figure 1(b) clearly shows that C-N@SiO2 has a distinct cavity structure, with a shell thickness of approximately 25 nm. Figures 1(c) and 1(d) show that the Pd/C-N@SiO2 material, after loading with Pd nanoparticles, still retains a clear cavity structure and good dispersion. Mapping (Figures 1(e) to (j)) characterizes the elemental composition of the material after metal loading, revealing that the Pd/C-N@SiO2 catalyst consists of C, N, O, Si, and Pd elements, where C is mainly distributed in the inner layer of the material shell, Si is primarily found on the surface of the material shell, and C and N elements are also present in the internal cavity of the material. The doping of N facilitates the anchoring of metal Pd, allowing for more metal Pd to be loaded. Additionally, Figure 1(j) shows that Pd has been successfully loaded onto the material.
2.1.2 N2 Adsorption-Desorption Curve Analysis
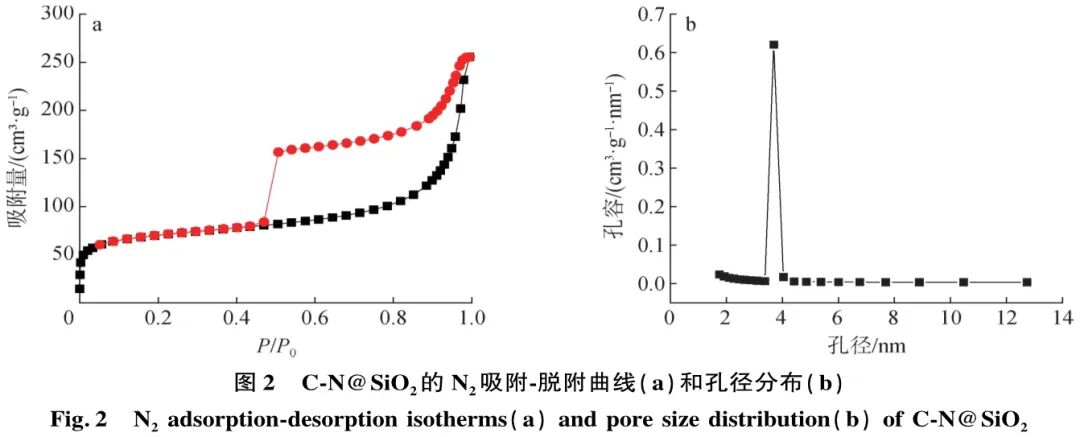
The C-N@SiO2 material was characterized using N2 adsorption-desorption methods, as shown in Figure 2(a). The N2 adsorption-desorption curve of the material is a standard type IV isotherm, and the curve shows a significant upward trend at both high P/P0 and low P/P0, indicating the presence of both micropores and mesopores in the material. The curve exhibits a distinct H3-type hysteresis loop at high P/P0 (0.4 ~ 1.0), indicating a perfect hollow structure and incomplete pore structure. The BET method calculated the BET specific surface area of the material to be 226 m2/g, and the BJH pore size distribution curve (Figure 2(b)) indicates an average pore size of 3.85 nm. The larger mesopores facilitate the entry of rosin molecules, and the larger cavity structure is more conducive to the disproportionation reaction of rosin.
2.1.3 XRD Analysis
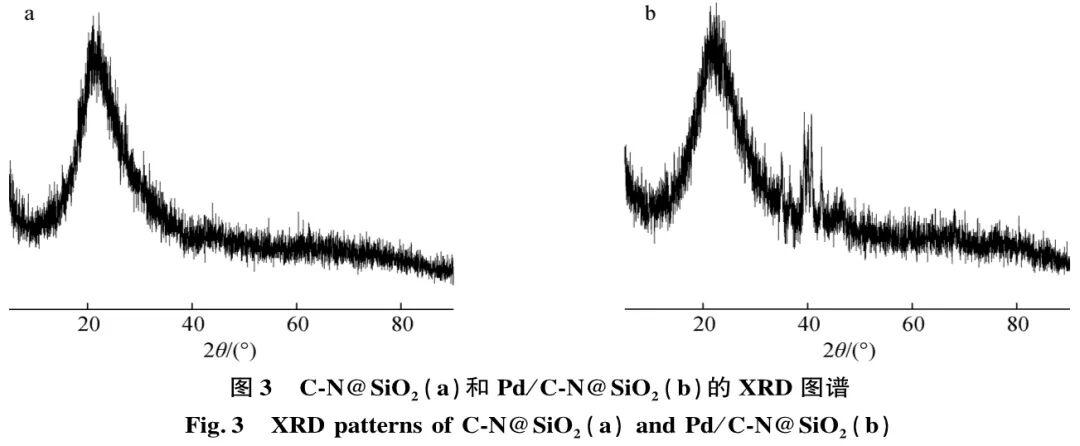
XRD characterization was performed on the materials before and after loading Pd metal, as shown in Figure 3. From Figure 3(a), it can be seen that the material without Pd metal loading shows a broad 002 diffraction peak at 23.5°, corresponding to the characteristic peak of disordered carbon structure. Figure 3(b) shows that the material has a distinct diffraction peak around 40°, corresponding to the characteristic peak of Pd, indicating that Pd has been successfully loaded onto the material.
2.1.4 XPS Analysis
XPS characterization was performed on the materials before and after loading Pd metal (Figure 4). From Figure 4(b), it can be seen that the Pd/C-N@SiO2 material contains C, N, O, Si, and Pd elements. The characteristic diffraction peak of Si2p is at 102.23 eV, C1s at 284.48 eV, Pd3d at 341.30 eV, N1s at 398.65 eV, and O1s at 531.09 eV, indicating that Pd has been successfully loaded onto the catalyst. The high-resolution XPS spectrum of C1s (Figure 4(c)) shows three characteristic peaks of functional groups, namely the C=C characteristic peak at a binding energy of 284.5 eV, the C—N characteristic peak at 285.5 eV, and the O—C=O characteristic peak at 287.1 eV. The high-resolution XPS spectrum of N1s (Figure 4(d)) shows three characteristic peaks of nitrogen, indicating that the nitrogen element in the Pd/C-N@SiO2 material consists of three different structural types of nitrogen atoms, with binding energies at 406.7, 400.4, and 397.6 eV corresponding to graphitic nitrogen, pyrrole nitrogen, and pyridine nitrogen, respectively. The high-resolution XPS spectrum of O1s is shown in Figure 4(e), with peaks at binding energies of 534.1, 533.5, and 532.9 eV corresponding to C=O, C—O, and COOH groups, respectively.
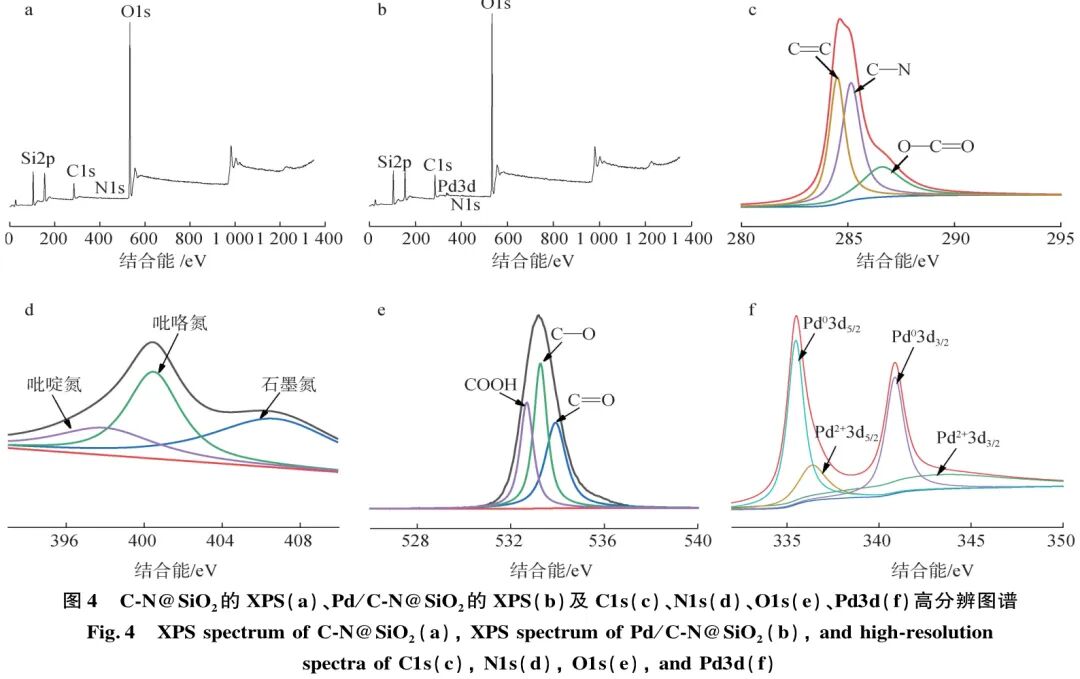
Using XPS for further valence state analysis of the loaded metal Pd. Figure 4(f) shows the high-resolution XPS spectrum of Pd3d, indicating that Pd exists in two valence states in the material. The peaks centered at binding energies of 335.7 and 341.0 eV correspond to the characteristic peaks of zero-valent metal Pd, specifically Pd03d5/2 and Pd03d3/2 diffraction peaks; the peaks centered at binding energies of 337.2 and 342.7 eV correspond to the characteristic peaks of divalent metal Pd, specifically Pd2+ Pd2+3d5/2 and Pd2+3d3/2 diffraction peaks. This indicates that not only is metallic Pd present in the catalyst, but also compounds of Pd2+. The integral calculation shows that the proportion of metallic Pd is 73.2%. The oxidized state of Pd may originate from by-products formed during the preparation process or may be due to oxidation of the sample during the drying and analysis process.
2.2 Optimization of Disproportionation Conditions for Rosin
2.2.1 Reaction Solvent
In a nitrogen atmosphere, under the conditions of 250 °C, 20 mg of catalyst, and a reaction time of 3.5 h, the effect of the ratio of water to 200# oil in the solvent on the rosin disproportionation reaction was investigated. Since rosin is not soluble in water, it was first dissolved in 200# oil, and then deionized water was added to introduce an aqueous phase environment. The total volume of water and 200# oil was controlled to be 15 mL, and the effect of the solvent oil volume on the rosin disproportionation reaction was examined, as shown in Figure 5(a). It can be seen from Figure 5(a) that when only 200# oil is used as the solvent, the catalyst, being amphiphilic, is not sufficiently dispersed in the solvent, resulting in limited contact with rosin and failing to provide the required three-phase steady-state environment for the reaction. Consequently, the GC content of dehydroabietic acid after the reaction is low, at 44.1%. As the amount of water gradually increases, the GC content of dehydroabietic acid shows a trend of first increasing and then decreasing. When the volume ratio of water to 200# oil is 3:12, the GC content of dehydroabietic acid reaches 53.3%, with a GC content of abietic acid at 0.7%. Further increasing the amount of water leads to a gradual decrease in the GC content of dehydroabietic acid, possibly due to an excess of water disrupting the three-phase steady-state environment and insufficient dissolution of rosin due to a lack of 200# oil. Therefore, 3 mL of water and 12 mL of 200# oil were chosen as the optimal reaction solvent.
2.2.2 Reaction Temperature
In a nitrogen atmosphere, under the conditions of 3 mL of water, 12 mL of 200# oil, 20 mg of catalyst, and a reaction time of 3.5 h, the effect of reaction temperature on the rosin disproportionation reaction was examined, as shown in Figure 5(b). The results indicate that at lower reaction temperatures, the GC content of dehydroabietic acid is low. As the reaction temperature increases, the activated state of rosin is more easily excited, leading to an increase in the GC content of dehydroabietic acid after the reaction. At a reaction temperature of 250 °C, the GC content of dehydroabietic acid reaches 53.3%, with the GC content of abietic acid being less than 1%. Further increases in temperature do not significantly change the GC content of dehydroabietic acid, thus 250 °C is selected as the optimal reaction temperature.
2.2.3 Reaction Pressure
Under the conditions of 3 mL of water, 12 mL of 200# oil, 20 mg of catalyst, a reaction temperature of 250 °C, and a reaction time of 3.5 h, the effect of reaction pressure on the rosin disproportionation reaction was examined, as shown in Figure 5(c). The results show that when the nitrogen atmosphere pressure in the reaction vessel is 0, the GC content of dehydroabietic acid reaches its highest at 53.3%, with a GC content of abietic acid at 0.7%. As the nitrogen pressure increases, the GC content of dehydroabietic acid decreases, while the GC content of abietic acid increases, which is unfavorable for the reaction. Therefore, a nitrogen atmosphere pressure of 0 is chosen as the optimal reaction pressure (gauge pressure of 0, meaning the pressure in the vessel is equal to atmospheric pressure, which is 1 atmosphere).
2.2.4 Reaction Time
In a nitrogen atmosphere, under the conditions of 3 mL of water, 12 mL of 200# oil, 20 mg of catalyst, and a reaction temperature of 250 °C, the effect of reaction time on the rosin disproportionation reaction was examined, as shown in Figure 5(d). The results indicate that as the reaction time increases, the GC content of dehydroabietic acid also increases. When the reaction time is 3.5 h, the GC content of dehydroabietic acid reaches 53.3%. Extending the reaction time further does not significantly change the GC content of dehydroabietic acid, thus 3.5 h is selected as the optimal reaction time.
2.2.5 Catalyst Amount
In a nitrogen atmosphere, under the conditions of 3 mL of water, 12 mL of 200# oil, a reaction temperature of 250 °C, and a reaction time of 3.5 h, the effect of catalyst amount on the rosin disproportionation reaction was examined, as shown in Figure 5(e). The results indicate that as the amount of catalyst increases, the GC content of dehydroabietic acid also increases. When the catalyst amount is 20 mg, the GC content of dehydroabietic acid reaches 53.3%, with the GC content of abietic acid being less than 1%. Further increases in catalyst amount do not significantly increase the GC content of dehydroabietic acid, thus 20 mg is chosen as the optimal catalyst amount.
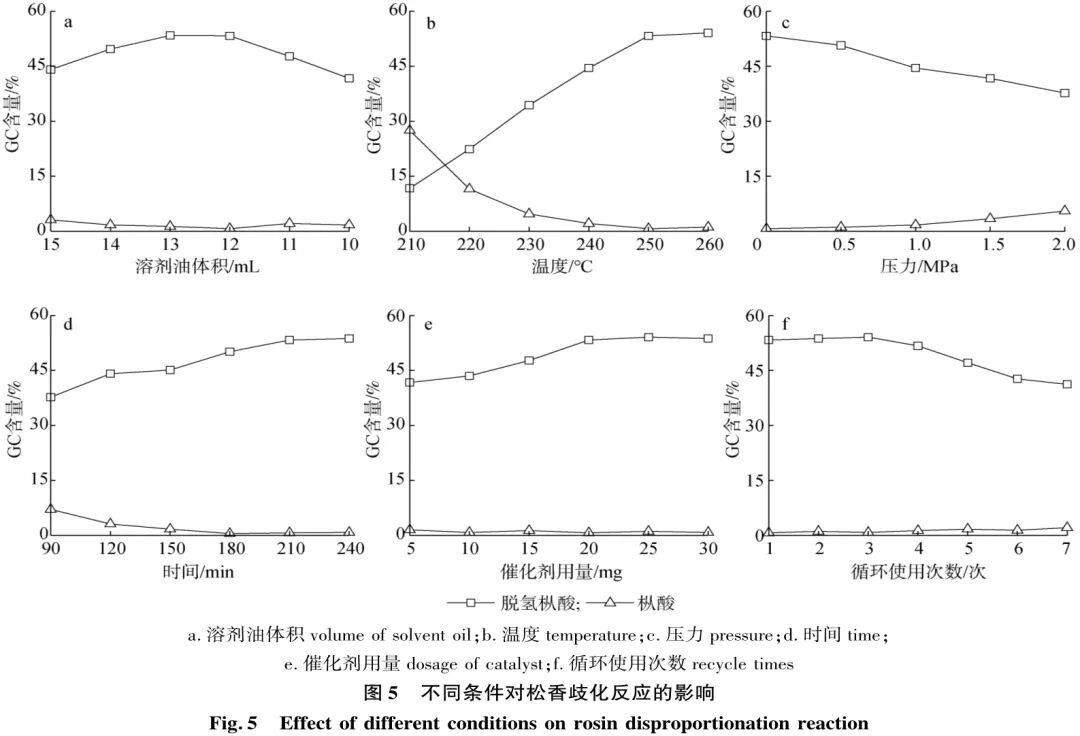
2.3 Catalyst Performance Analysis
2.3.1 Catalyst Reusability
Under the optimized conditions of Section 2.2, after the reaction, the mixture of catalyst and reaction products was removed, and the catalyst was separated by centrifugation. The catalyst was then washed several times with deionized water and ethanol, and placed in a vacuum drying oven at 60 °C for 4 h. Fresh catalyst was added to replace the lost portion, and the catalyst was reused for the rosin disproportionation reaction. The effect of the number of catalyst reuse cycles on the rosin disproportionation reaction is shown in Figure 5(f). It can be seen from Figure 5(f) that when the catalyst is reused for the 6th time, there is a noticeable decline in catalytic performance. The reason may be due to the harsh reaction conditions causing the active components of the catalyst to fall off. ICP analysis revealed that the mass fraction of Pd in the catalyst decreased from 1.86% before use to 1.27% after 5 cycles of reuse, and it cannot be ruled out that the active components of the catalyst were oxidized during the reuse process. The catalyst can be efficiently used at least 5 times in the rosin disproportionation reaction, at which point the GC content of dehydroabietic acid is 47.1%, indicating that the catalyst has good stability and reusability.
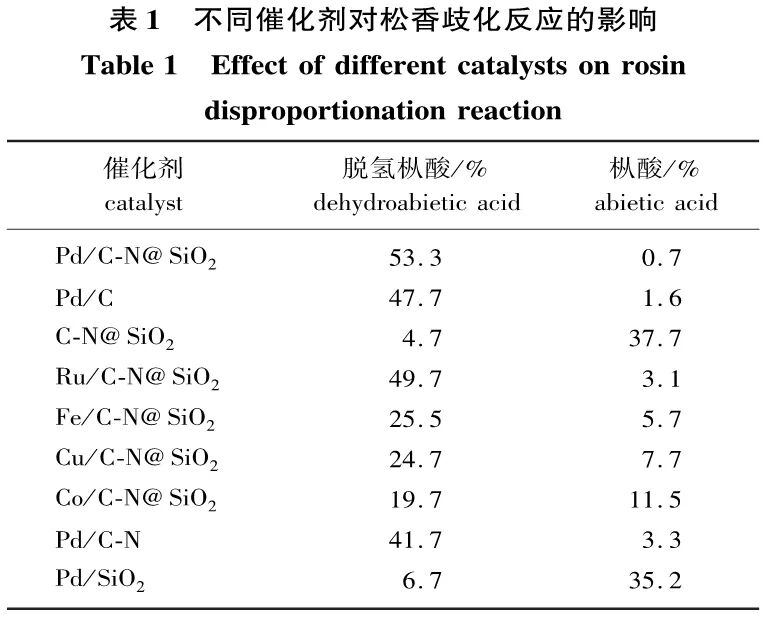
2.3.2 Catalyst Performance Comparison
As noted in Section 2.2, the optimal reaction conditions are a nitrogen atmosphere, a water/oil volume ratio of 3:12, a reaction temperature of 250 °C, a reaction time of 3.5 h, and a catalyst amount of 20 mg. Under these optimized conditions, the catalyst without metal loading, catalysts loaded with different metals (Pd, Ru, Fe, Cu, Co), Pd/C-N, Pd/SiO2, and Pd/C catalysts were used for the rosin disproportionation reaction. The comparative experimental results are shown in Table 1. It can be seen from Table 1 that the catalyst without metal loading has poor catalytic performance in the rosin disproportionation reaction, indicating that C-N@SiO2 cannot be directly used for the rosin disproportionation reaction. After loading with Pd metal, the catalytic activity of Pd/C-N@SiO2 exceeds that of Pd/C catalysts and non-amphiphilic catalysts (Pd/C-N, Pd/SiO2), due to the amphiphilic nature of the support material enhancing the dispersion of the catalyst in the solvent, which is more favorable for the reaction. The catalytic performance of non-precious metals (Fe, Cu, Co) is also not ideal, as their catalytic activity is lower than that of Pd. The catalyst Ru/C-N@SiO2 with loaded Ru metal also shows lower catalytic activity than the Pd-loaded catalyst, thus the Pd/C-N@SiO2 catalyst is preferred for the rosin disproportionation reaction.
3 Conclusion
3.1 A novel amphiphilic hollow nanomaterial C-N@SiO2 was designed and prepared, and the Pd/C-N@SiO2 catalyst was obtained through an impregnation method. The catalyst was characterized using SEM, TEM, N2 adsorption-desorption, XPS, XRD, and other methods to analyze its morphology, structure, elemental composition, etc. The results indicate that this catalyst has a large cavity structure and large pore size, which is beneficial for the entry of rosin molecules and the progression of the disproportionation reaction; nitrogen doping facilitates the anchoring of metal Pd, and the amphiphilicity of the material enhances the stability and dispersion of the catalyst, confirming that Pd has been successfully loaded onto the C-N@SiO2 material.
3.2 The Pd/C-N@SiO2 catalyst was applied to the rosin disproportionation reaction, and the reaction conditions were optimized. The results show that under a nitrogen atmosphere, with a solvent water/oil volume ratio of 3:12, a reaction temperature of 250 °C, a reaction time of 3.5 h, and a catalyst amount of 20 mg, the GC content of dehydroabietic acid in the reaction product reaches 53.3%, with the GC content of abietic acid being less than 1%. The catalytic activity does not show significant decline after 5 reuse cycles. Meanwhile, the catalytic effect of this catalyst is superior to that of commercially available Pd/C catalysts, indicating its potential industrial application value.
「ENDING」
Journal Introduction
The Journal of Chemistry and Industry of Forest Products (founded in 1981) is supervised by the State Forestry and Grassland Administration and co-sponsored by the Institute of Forest Products Chemistry and Industry of the Chinese Academy of Forestry and the Forest Products Chemistry and Chemical Engineering Branch of the Chinese Forestry Society. It is an academic journal in the national forest products chemical industry, with Academician Jiang Jianchun as the editor-in-chief. The scope of reporting includes the chemical processing and utilization of renewable wood and non-wood biomass resources, with research fields covering biomass energy, biomass chemicals, biomass new materials, natural active components of biomass, and pulping and papermaking, among others. The journal mainly includes the latest research results in areas such as rosin chemistry, biomass energy chemistry, biomass carbon materials, bio-based functional polymer materials, adhesive chemistry, chemical utilization of extracts from forest plant resources, environmental protection engineering, and research and design of integrated forest and paper chemical engineering equipment. This journal is published bimonthly, in large 16 open format, with ISSN 0253-2417, CN 32-1149/S, CODEN LHYGD7. It is publicly distributed both domestically and internationally, with domestic mailing code 28-59; and foreign distribution code Q5941.
This journal is currently indexed by various international databases, including the American Chemical Abstracts (CA Core), the Dutch Abstracts and Citation Database (Scopus), the Ulrich’s International Periodicals Directory, the British Commonwealth Agricultural and Biological Science Abstracts (CAB Abstracts), the British Global Health, the Royal Society of Chemistry Series Abstracts (RSC), the Russian Abstract Journal (PЖ), the Japanese Science and Technology Agency Database (JST), EBSCO, and others; it is also included in the core database of the Chinese Science Citation Database (CSCD), Peking University Chinese Core Journals, Chinese Science and Technology Core Journals, RCCSE Chinese Authoritative Academic Journals (A+), Chinese Agricultural and Forestry Core Journals (2020 edition) (A category), the World Journal Impact Factor Index (WJCI) report (2022), the China Journal Full-text Database, the Comprehensive Evaluation Database of Chinese Academic Journals, Wanfang Data – Digital Journal Group, the Chinese Science and Technology Journal Database, the Chinese Core Journals (selected) database, and the China Academic Journal Abstracts, among others. It has been classified as a high-quality scientific and technological journal in the agricultural and forestry field in China, with a T3 level and as a key journal in forestry and grassland science and technology (2022-2025). It was awarded the title of “China’s Excellent Science and Technology Journal” in 2008 and 2011, and has received the Jiangsu Province Science and Technology Journal “Golden Horse Award” for outstanding team, excellent journal, outstanding contribution, and top ten excellent journals.
Address: No. 16, Suojin Wucun, Nanjing, Jiangsu Province, Institute of Forest Products Chemistry and Industry
Postal Code: 210042
Phone: 025-85482493
Email: [email protected]
Website: https://journals.caf.ac.cn/lchxygy
Scan the QR code to follow us for more information
