Antibody-drug conjugates (ADC) are formed by linking monoclonal antibodies targeting specific antigens with small molecule cytotoxic drugs through linkers, combining the powerful killing effect of traditional small molecule chemotherapy with the tumor targeting ability of antibody drugs. Since ADCs first entered clinical trials in the mid-1990s, they have developed into a very successful oncology platform over nearly 30 years.
As of December 2021, the U.S. Food and Drug Administration (FDA) has approved 14 ADC drugs. Compared to new immunotherapy targets based on antibodies, more ADCs with new targets (TF, Nectin-4, CD19, Trop-2, BCMA, CD79b, etc.) have been approved in recent years. However, ADCs currently under development still face many challenges, which arise at an alarming rate and largely hinder clinical progress.
Based on this, this article summarizes the successes and failures experienced in ADC clinical trials to gain faster and safer pathways for the development of more ADC drugs in the future.
Selecting Appropriate Subjects
In Phase I studies, many designs aim to select patients who are almost perfect and most likely to respond, neglecting the established goal of determining a safe dose for the tumor patient population in Phase I. In fact, the eligibility criteria are often too restrictive, making it slow to find study patients, and the results obtained are often not applicable to the typical patients who genuinely need them. Therefore, qualified selection criteria should reflect the “real” patients needing clinical trials.
Some Patients with Organ Dysfunction Should Be Allowed
The population entering Phase I studies typically has physical and biochemical symptoms of refractory and advanced disease. Moreover, these cancer patients are often elderly and may have hypertension, diabetes, and chronic obstructive pulmonary disease, all associated with certain organ dysfunctions.
If preclinical toxicology does not show liver or kidney toxicity, the study protocol should allow for some degree of dysfunction in these organs. It is generally recommended that serum creatinine levels can be up to and including 1.5 times the normal upper limit (ULN), and liver transaminases can be up to 3 times the ULN, without liver metastases. For patients with liver metastases, it is recommended to use 5 times the ULN.
Do not exclude patients with elevated alkaline phosphatase, as alkaline phosphatase may originate from the bone or liver, and if bilirubin and liver transaminases are acceptable, there is almost no practical impact on tolerance. Since patients with prostate cancer, lung cancer, and breast cancer have a high rate of bone metastasis, excluding them unnecessarily due to elevated alkaline phosphatase may be counterproductive.
Do not exclude patients based on serum albumin levels, as there is no evidence that an albumin level of 2.8 is more clinically significant than 3.0 in clinical research. Since albumin levels are negatively correlated with tumor burden, this may inadvertently select patients with lower tumor burdens. This restriction will lead to significant bias.
Avoid Unjustified Special Criteria
One study arbitrarily excluded patients with oxygen saturation below 93% in ambient air. This criterion did not specify whether it was at rest or during activity. Although the rationale is to reduce the risk of serious pulmonary toxicity, there is no data from preclinical toxicology studies on lung injury from this molecule or payload. Furthermore, there is no known evidence that pre-study oxygen saturation can predict ADC pulmonary toxicity. However, this exclusion criterion certainly eliminated many lung cancer patients, potential chronic obstructive pulmonary disease patients, and patients with lung metastases.
Anticoagulants and a certain degree of coagulation parameter abnormalities should be allowed. Thromboembolic events are common in many cancer indications, and the use of factor Xa inhibitors can effectively control and prevent thromboembolic events. Unless thrombocytopenia is predicted to be related to the selected ADC toxicity, restricting patients from using anticoagulants or requiring normal coagulation parameters will exclude some excellent candidates.
Moreover, a decrease in absolute lymphocyte count (ALC) is a common manifestation in patients with advanced cancer. This laboratory finding has little clinical impact on most patients, and restricting patients with low ALC from entering the study usually offers no benefit. The strongest evidence against restricting ALC comes from the initial clinical trials of pembrolizumab. Even in this very successful immunotherapy, no ALC standard was used, so it should not be included in ADC development protocols.
Do Not Exclude Previous ADC Treated Patients
This exclusion criterion has been observed in some newer protocols, which is neither consistent with contemporary drug development standards nor increasingly realistic, as many ADCs have been approved or are in clinical development in recent years.
The typical reason for excluding patients who have previously been treated with ADCs is to reduce the risk of acquired resistance to the latest ADC by excluding patients who have previously been exposed to ADCs. However, the reasons for patients not responding to ADCs are multifactorial, including too low a dose level received, low expression levels of target antigens in tumors, unstable linkers, or inherent resistance of the tumor to the payload. Additionally, excluding patients who have received the same payload may not be entirely effective, as responses have occasionally been observed in patients with ADCs that have similar payloads in the past.
New therapies must address unmet needs of patients, and the most valuable and fastest route to approval is based on the benefits of patients who have previously failed treatment. The accelerated approval of trastuzumab deruxtecan (Enhertu®) for HER2-positive metastatic breast cancer is a good example. This study specifically included HER2-positive breast cancer patients who had failed treatment with trastuzumab-emtansine (Kadcyla®). The significant response rate and prolonged response duration led to accelerated approval. If the study of trastuzumab deruxtecan had excluded previous ADC treatment, it would likely have required a randomized trial comparing it with trastuzumab emtansine for conventional approval.
MTD is typically derived from safety data from Phase I trials. However, in reality, drug toxicity only becomes apparent after several cycles, some of which can become increasingly severe. For example, some promising ADCs are associated with life-threatening pneumonia that occurs only after multiple cycles. Therefore, recommended doses should be determined after careful analysis of multiple patients, multiple cycles, and dose reductions and delays. Spending some extra time in Phase I to refine the recommended dose is much better than having to go back and publicly announce dose reductions in the study later.
Moreover, many clinical trials suffer from the issue that the principal investigator is merely nominal, and patients are assessed by a series of researchers, residents, and sub-investigators. These individuals may have varying degrees of familiarity with common toxicity standards (NCI CTC). The staff caring for patients often do not consult specific definitions related to toxicity grading but use a common arbitrary description of mild (Grade 1), moderate (Grade 2), severe (Grade 3), and life-threatening (Grade 4) to describe toxicities beyond hematological and chemical features. This can lead to underreporting of drug toxicity, especially non-hematological toxicity. Having researchers who are very familiar with NCI CTC and have extensive experience in toxicity classification is key to accurately collecting safety data and successfully determining recommended doses.
Finally, there is evidence that toxicity recurs due to weight-based dosing. The vast majority of ADCs are dosed in mg/kg or mg/m2, yet there is little scientific evidence regarding this in oncology, let alone for ADCs. Basic pharmacological principles tell us that antibodies or other macromolecular therapeutic agents are initially confined to the vascular compartment. However, because adipose tissue is not well vascularized, the vascular volume does not increase proportionally with total weight. Increasing weight-based dosing in obese or overweight patients can disproportionately increase plasma concentrations, leading to increased toxicity. With the rise of obesity in the Western world, this is a reality that must be considered in clinical trials. Additionally, some patients may develop third-space fluid (ascites, edema, and pleural effusion).
In this regard, mirvetuximab soravtansine is a good example. In the early clinical development of this drug, dose-limiting keratopathy was a non-target tumor adverse event that occurred more frequently at higher doses when the drug was dosed in mg/kg. Despite numerous efforts, this keratopathy could not be alleviated by local measures such as lubricants or steroid eye drops. This posed challenges for the feasibility of later development. Subsequently, researchers conducted detailed pharmacokinetic analysis and modeling to determine the relationship between Cmax and the severity of keratopathy. Further studies indicated that dosing needed to use an adjusted ideal body weight formula (AIBW) to achieve a safe and effective plasma concentration range across a range of patients. This dosing method was further validated in a broader population of ovarian cancer patients, significantly reducing keratopathy from 40% to 10% while maintaining anti-tumor activity.
Since some ADCs have a therapeutic index smaller than non-ADC antibody therapies, the relationship between dose, dosing formula, clinical pharmacokinetics, and toxicity should be carefully analyzed to determine the optimal dosing method.
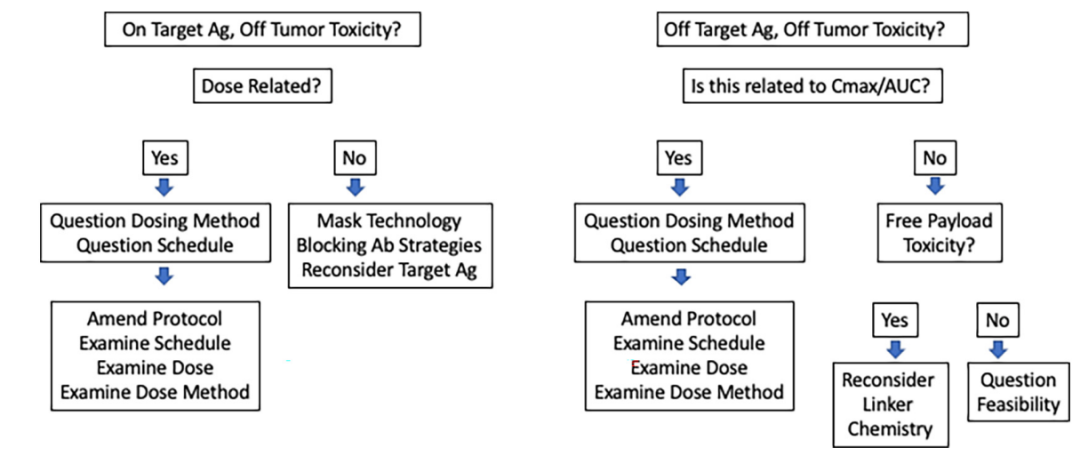
Typically, ADC Phase I studies select only one dosing regimen. The conventional dosing schedule for new drugs usually comes from cytotoxic drugs, given once every 3 weeks, as this is close to the hematological recovery time. In the era of antibodies, doses are increasingly based on expected clearance rates and half-lives, leading to dosing schedules of every 2 to 4 weeks. Therefore, there is no reason to adopt the schedules of cytotoxic drugs or antibodies for ADC drugs. Perhaps a better option would be to select schedules based on tolerance, pharmacokinetics, and/or target antigen turnover and target antigen regeneration kinetics.
Gemtuzumab ozogamicin (Myelotarg®) is an excellent research case in ADC development. Gemtuzumab ozogamicin was the first ADC approved by the FDA, approved in 2000, voluntarily withdrawn in 2010, and then reapproved in 2017. The initial plan for this ADC was 9mg/m2 every 2 weeks. In the earliest studies, a disproportionate increase in plasma drug concentration above the saturation level of CD33+ was observed, leading to a saturated pharmacokinetic pattern, with a nonlinear relationship between dose and plasma concentration. This accumulation of the drug in plasma, above the levels necessary to saturate CD33+ target cells, was at least partly responsible for many of the toxicities observed, including bone marrow suppression, acute liver toxicity, and hepatic veno-occlusive disease post-transplant. In 2010, after subsequent confirmatory trials failed to validate its clinical efficacy and demonstrate its safety, Gemtuzumab ozogamicin was voluntarily withdrawn from the market.
Later studies determined that the kinetics of CD33 expression conversion on AML target cells was approximately every 3-4 days. Subsequently, researchers provided a new regimen of low-dose 3mg/m2 every 3 days for 3 doses. Using a split dosing regimen reduced the peak and total plasma concentrations of Gemtuzumab ozogamicin to the expected dose ratio range. Clinically, this resulted in greater tolerability and anti-tumor activity, and in 2017, the FDA reapproved Gemtuzumab ozogamicin.
The primary strategy for developing ADC biomarkers is to identify and select cancer patient subgroups that are most likely to respond to ADCs, thus achieving a high rate and durable response, ensuring regulatory approval.Implementing this strategy is often the biggest issue in biomarker development.
In Early Studies, Accept as Many Tumor Patients as Possible Who May Express the Target
This seems counterintuitive to the overall strategy just described, but it is one of the most common mistakes in early development that can significantly slow clinical execution. To better understand the pre-screening issues of target antigen presence, it is essential to understand the real process of tissue collection and analysis in clinical research.
Many patients do not have easily accessible archived tissues. Furthermore, obtaining tissues for trial screening requires a lengthy process, and even the fastest turnaround time for analysis usually still takes 6 weeks to obtain results. Now imagine a patient with advanced malignancy who is unresponsive to standard treatment and is considering participating in a clinical trial. Will they wait for the pre-screening results? Or will they go for another clinical trial that does not require pre-screening? The answer is obvious for most and reflects how patients enter clinical trial centers.
Do Not Wait for Perfect Assays
Biomarker development is complex. Although ADCs have antibodies as scaffolds, the antibodies selected for treatment may not be satisfactory for immunohistochemistry (IHC). It is very common to select different antibody candidates for IHC biomarkers. The usual recommendation is that clinical trials and biomarker development should often be conducted in parallel.
Do Not Overemphasize Target Antigen Expression Levels
It must be acknowledged that tumor cells must express the target antigen to exert anti-tumor activity for ADCs. However, even high expression does not guarantee anti-tumor activity, nor does low expression guarantee a lack of activity. ADCs may fail to induce tumor cell death due to many factors independent of tumor cell expression.
Contrary to intuition, some patients with low target antigen expression at initial diagnosis have observed responses. This may be due to the age, quality of tissue samples, and specificity of the assays used, or it may be due to increased target antigen expression as the disease progresses. Therefore, the most reasonable approach in early development is not to rely solely on the confirmed expression of target antigens in early clinical trials to select patients but to utilize expansion-phase data to determine the relationship between expression and response.
Furthermore, not every ADC requires biomarkers for patient selection. For many hematological malignancies, target cells are of a specific lineage; thus, ADCs can target both normal and malignant cells. Examples in this regard include CD33+, CD22, CD79b, and CD30.
Finally, if biomarkers unnecessarily or arbitrarily prevent patients from receiving potentially beneficial treatments, this may limit future commercial opportunities.
Do Not Change Assays or Endpoints During the Study
This may sound self-evident, but recent experiences suggest otherwise. A recent example of this error is mirvetuximab soravtansine, an ADC targeting folate receptor α (FRa) in platinum-resistant ovarian cancer. In the Phase II study, favorable selection criteria for high FRa expression were used, observing promising anti-tumor activity in platinum-resistant ovarian cancer.
Unfortunately, the study later changed the patient selection parameters for the assay, including patients with moderate FRa expression. This led to the inability to achieve the primary endpoint of improved progression-free survival (PFS). Post-hoc analysis found that the subset of patients with high expression (FRa) had statistical significance similar to those in the early study.
Until recently, many clinical pharmacology steps required for regulatory approval during ADC development were not outlined separately from drugs or biologics, raising the question of whether ADCs should be viewed as cytotoxic drugs or closer to antibodies. This uncertainty has led to questions about whether organ dysfunction studies, drug interaction studies, and comprehensive QTc studies are necessary.
Recently, the FDA’s Center for Drug Evaluation and Research (CDER) and the Center for Biologics Evaluation and Research (CBER) jointly released draft guidelines to assist in the clinical development of ADCs. These very practical recommendations should help industry researchers determine which clinical pharmacology studies are needed. It can also be argued that the release of guidelines further demonstrates that ADCs are a platform unto themselves, transcending cytotoxic and antibody development.
Currently, there are more than 20 new ADC drugs in early development, with many more in late preclinical research stages. The prospects for ADC therapies to improve cancer patients’ outcomes are very promising. The key to success often lies in learning from others and avoiding their mistakes. It is hoped that this article can provide some useful and practical approaches to ensure the success of clinical development, closely reflecting the patient population, and these lessons will provide the best path forward.
References:
1. Antibody drug conjugates: The dos and don’ts in clinical development. Pharmacol Ther. 2022 Jun 20;240:108235.
Disclaimer
