By | Little Medicine Monster
Global ADC Pipeline Status
As of December 6, 2023, a total of 1166 ADC pipelines have been developed globally, with 524 pipelines under research and 16 approved drugs (including two TDM-1 biosimilars). The abandonment rate of pipelines is as high as 54% (1166-524-16/1166).
Among the 524 pipelines under research, over 95% are developed for anti-tumor treatment (499/524); the development targets are concentrated, with the top 5 targets being HER-2 (46), Trop-2 (25), Claudin18 (17), HER-3 (14), and Nectin-4 (12). Except for Claudin18 and HER-3, the other targets have already been developed into drugs using the ADC modality; the combined ADC pipelines in the US and China account for over 86%; there is 1 pipeline in the registration phase, 13 in clinical phase III, 58 in clinical phase II, and 100 in clinical phase I, with the majority of the remaining pipelines in the preclinical stage (352).
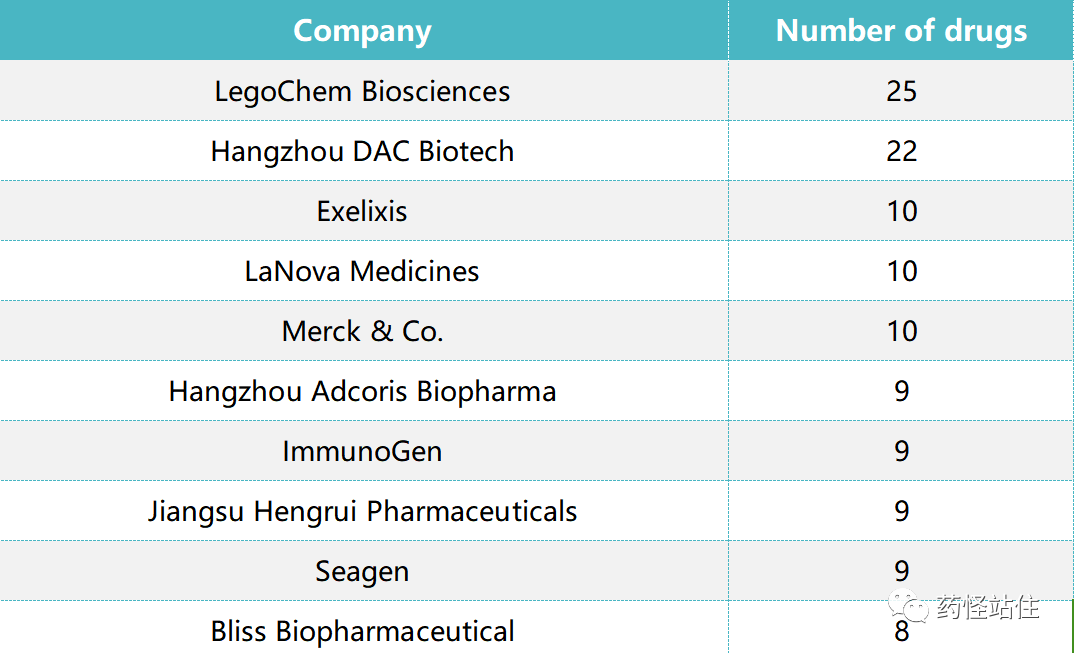
The Top 10 Companies with the Most ADC Pipelines, with Chinese Companies Holding Half of the MarketSouth Korea’s LegoChem Biosciences leads with 25 pipelines; Mucosis follows closely with 22 pipelines; other notable Chinese companies include Livzon, Aikangruisi, Jiangsu Hengrui, and Bailliskang.
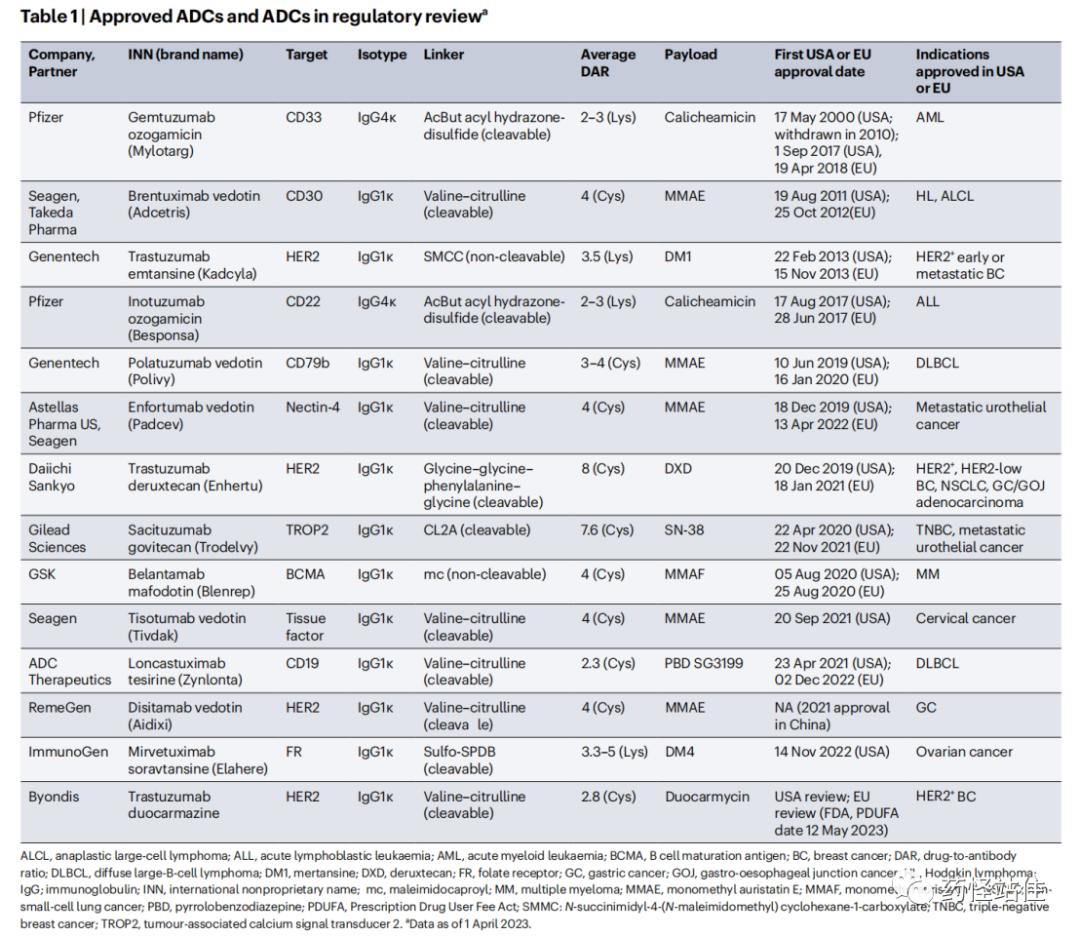
A Total of 16 ADC Drugs Approved Globally, excluding the two TDM-1 biosimilars (Padynex and Ujvira), a total of 14 drugs have been approved.
Current Status of the Global ADC Market
For the 16 approved ADCs, the total sales in 2022 amounted to approximately 6 billion USD, with the solid tumor market at 3.7 billion USD and the hematological tumor market at 2.36 billion USD.
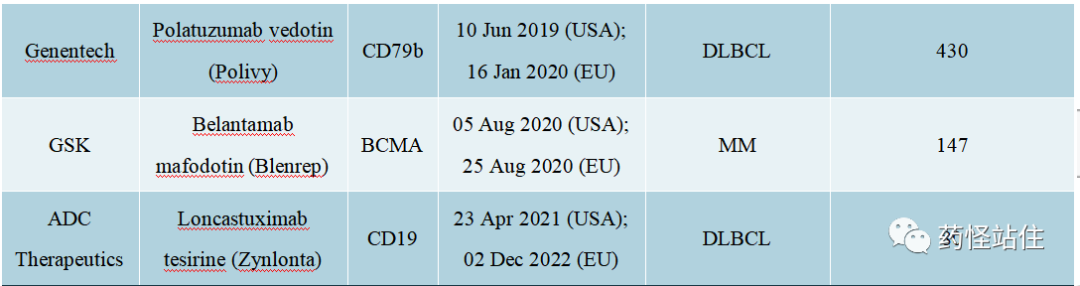
Performance of 6 Hematological Tumor ADCs in 2022

Performance of 8 Solid Tumor ADCs in 2022
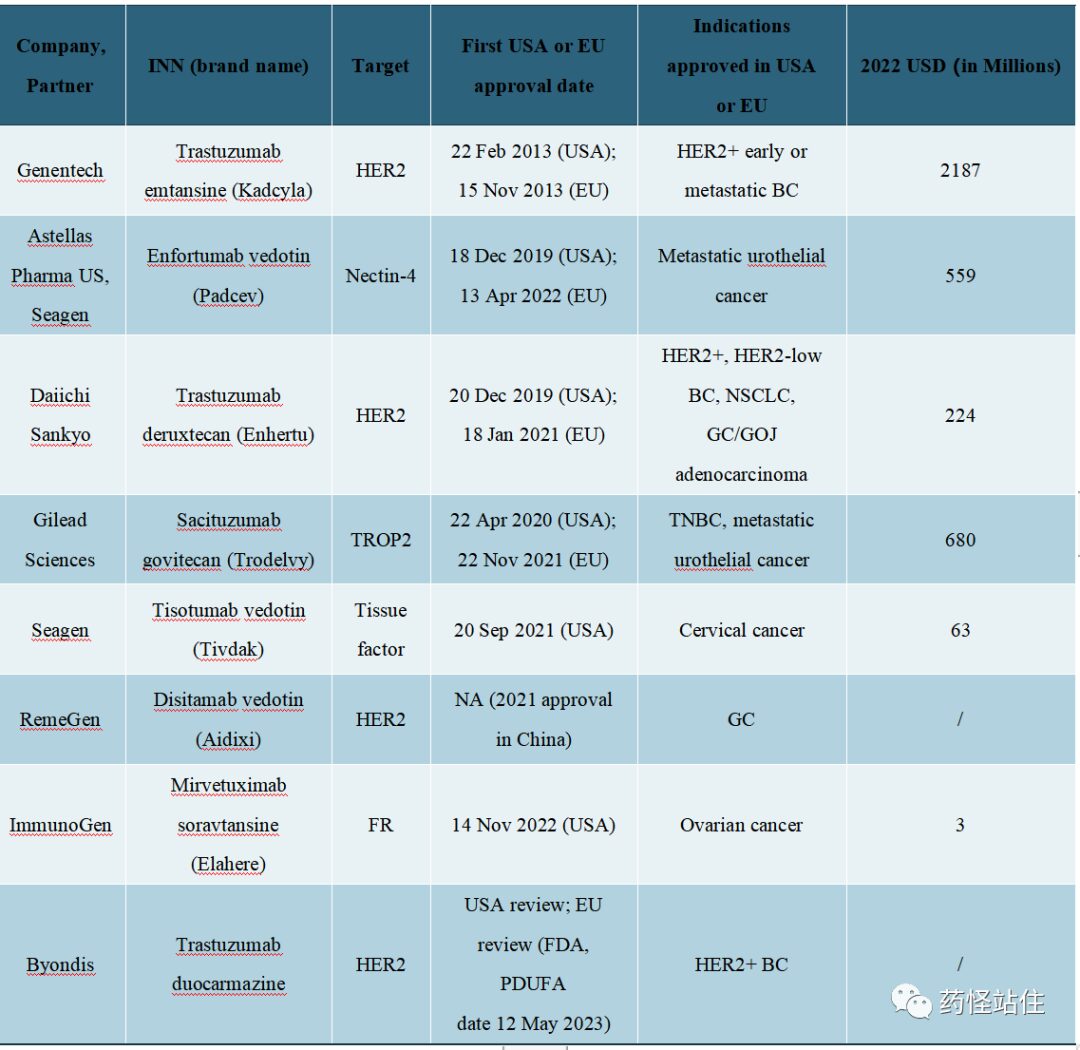
8 Approved ADCs for Solid Tumors
A total of 8 drugs have been approved for solid tumors, with 4 drugs targeting HER-2 ADCs, while other targets include Nectin-4, Trop-2, TF, and FRa; all monoclonal antibodies used are of the IgG1 class, and the payloads are divided into two types: microtubule inhibitors and topoisomerase inhibitors; except for TDM-1 which has an uncleavable linker, the others all have cleavable linkers, possessing a bystander effect; the DAR value ranges from 3.5 to 8.
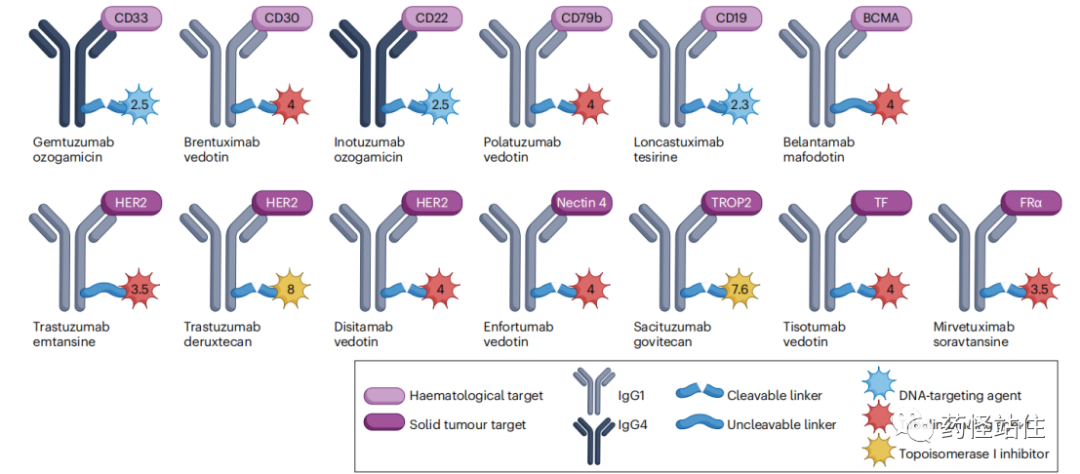
Roche’s Kadcyla is the world’s first ADC approved for the treatment of solid tumors, targeting HER-2, which was first approved by the FDA in 2013 for HER-2 positive breast cancer, with global sales reaching 2.187 billion USD in 2022, accounting for 59.1% of the solid tumor ADC market.
After a 6-year silence period, the solid tumor ADC market began to flourish in 2019, with the approvals of Padcev, Enhertu, Trodelvy, Tivdak, Aidixi, and Elahere. Except for the two fastest drugs, Kadcyla and Padcev, which have early (adjuvant HER2-positive breast cancer) and advanced first-line UC indications, the other ADCs currently have indications in late-stage lines. As clinical trials progress, some ADCs may have the potential to challenge first-line indications in the future, further expanding the market. The subsequent articles will detail the development history, clinical strategies, and future prospects of each solid tumor ADC.
2. Dumontet C, Reichert JM, Senter PD, Lambert JM, Beck A. Antibody-drug conjugates come of age in oncology. Nat Rev Drug Discov. 2023 Aug;22(8):641-661. doi: 10.1038/s41573-023-00709-2. Epub 2023 Jun 12. PMID: 37308581.
Every article is carefully crafted by the author.All materials in the text are sourced from primary data, striving to cite high-impact literature, with most charts being self-organized, primarily relying on the Informa database, Clinicaltrials database, clinical trial registration websites, Zhihuiya, Yaowang, Yiyuan Magic Cube database, Insight database, various national regulatory agency websites, international conference websites, and the official websites of various companies.Hate plagiarism and content laundering; if you like the article, welcome to revisit, share, and follow.
The public account content will undergo a series of reforms. The author relies solely on personal efforts, completing the writing and operation of the “Medicine Monster Stand Still” public account alone, striving to continuously provide valuable, attitudinal, and warm content, aiming to provide at least 1-2 articles per week. In addition to the previous in-depth single articles, we are preparing to launch high-quality series articles and emotional articles, aiming to bring academic value and emotional value to everyone. Commercial cooperation is accepted appropriately.
Interested friends can add me on WeChat to join the group, please note (company and position), and the group will subsequently push a series of article references, as well as learning materials irregularly.







