
1
Introduction
Antibody-drug conjugates (ADC) are an emerging technology that selectively targets cancer cells to overcome the limitations of chemotherapy. ADC binds to antigens, especially those overexpressed on the surface of cancer cells, reducing side effects caused by nonspecific targeting and improving the therapeutic index. The ideal efficacy of an ADC is entirely dependent on several physicochemical factors, such as binding sites, molecular weight, linker length, steric hindrance, half-life, conjugation methods, etc. As of February 2023, there are 15 ADCs approved globally by the FDA, with over 100 ADCs undergoing clinical trials. However, designing an ideal ADC remains a significant challenge. Therefore, a profound understanding of the key components and their characteristics of ADC will aid in developing ADCs with higher safety and therapeutic indices.
2
Key Components of ADC
ADC consists of antibodies, linkers, and cytotoxic payloads, with each component playing a crucial role. An ideal ADC should effectively reach the target site without releasing any off-target payloads and should be able to kill cancer cells without affecting normal healthy cells. To develop a successful ADC with maximum efficacy, all these components should be considered, such as the selection of antigens, antibodies, toxic payloads, and linkers.
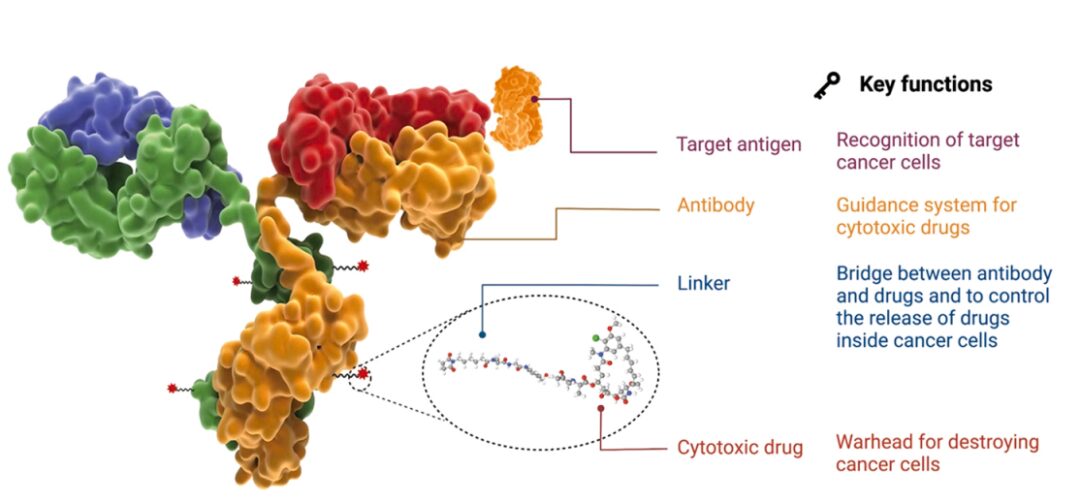
Antigen Selection
The target antigen helps distinguish between cancer cells and normal cells, thereby reducing off-target toxicity. Therefore, selecting the appropriate target antigen is the first step in developing an ideal ADC. The ideal antigen must have certain characteristics, such as being overexpressed on the surface of cancer cells compared to healthy cells; secondly, the target antigen should not have a secreted form to avoid ADC binding outside the tumor; finally, the target antigen should have internalization efficacy to bring in the ADC’s payload. Currently, among approved and clinical ADCs, the most commonly used targets in hematological and solid tumors include CD33, CD30, CD22, BCMA, CD19, CD79b, and HER2, Nectin-4, Trop-2, EGFR, TF, etc.
Antibody Selection
In an ideal ADC, the antibody is the key carrier that specifically binds to the target antigen, and it must have high affinity for the target antigen and low immunogenicity. Additionally, the antibody should maintain a long plasma half-life and have rapid internalization capabilities. Due to the abundance of antibody types and the ability to initiate immune effectors, IgG is the most common type of antibody used in ADC development. Among the different subclasses, IgG1 is the most commonly used antibody subclass in ADC development.
Internalization is another major factor affecting ADC efficacy, and the dissociation constant (Kd) is a key factor influencing ADC internalization into cancer cells. Ideally, Kd should be high to allow effective penetration and uniform distribution throughout the tumor tissue. Furthermore, the molecular weight of the antibody is another key factor affecting ADC penetration into tumor tissue. Due to the large molecular weight of IgG, it becomes a challenging task for ADC to penetrate blood vessels and reach tumor sites. Therefore, considering smaller-sized antibody types may be beneficial for the development of an ideal ADC.
Linker
The linker serves as a bridge between the antibody and the payload, primarily contributing to the stability and efficacy of the ADC. Furthermore, the release of the payload largely depends on the type and nature of the linker. An ideal linker should be highly soluble in water, preventing the formation of aggregates of the ADC and premature release of the payload during systemic circulation. Generally, all three components of the ADC, such as the antibody, linker, and payload, can be modified to obtain a stable and effective ADC. The three main factors affecting linker stability and payload release are binding sites, steric hindrance, and linker length.
Payload
The payload is the warhead of the ADC, which is a drug with high cytotoxicity. The ideal ADC payload should have high potency, stability during metabolism or breakdown in systemic circulation, and high solubility. Additionally, it should possess functional groups for conjugation and membrane permeability.
Due to the lysosomal barrier and the complexity of the tumor microenvironment, the amount of cytotoxic drug that can reach the target is very small; therefore, low IC50 payloads should be selected. For example, the IC50 of DNA damaging agents is generally at the picomolar concentration level, while microtubule inhibitors are in the nanomolar range. Regarding stability, the payload should remain stable against any chemical reactions during systemic circulation and manufacturing processes. If the payload is unstable under lysosomal conditions, it should be separated before reaching the cell surface or entering the cell.
3
Conjugation Methods of ADC
The conjugation method and sites are also critical factors in designing an ideal ADC. They can regulate the location and rate of payload release, which ultimately relates to the safety and efficacy of the ADC.
Conjugation with Endogenous Amino Acids
One of the most common conjugation methods is utilizing lysine residues of the antibody, where the nucleophilic NH2 group of the amino acid reacts with the electrophilic N-hydroxysuccinimide (NHS) group on the payload. Although the reaction is simple, the high abundance of lysine residues can lead to the formation of uneven mixtures of many ADCs distributed randomly.
Disulfide Rebridging Strategy
IgG antibodies contain four interchain disulfide bonds, two linking light and heavy chains, and two located in the hinge region connecting the two heavy chains, which maintain the integrity of the monoclonal antibody. Another classic bioconjugation pathway explores the role of these cysteines as payload connection points. The reduction of the four disulfide bonds typically generates eight thiol groups that can react with maleimide linkers, resulting in ADCs with a DAR of 8.
Glycan Conjugation
Since IgG is a glycoprotein, it contains an N-glycan at the N297 position of each heavy chain in the Fc fragment, and this glycosylation can serve as an attachment point for connecting the payload. The distant positioning of the glycan from the Fab region reduces the risk of damaging the antibody’s antigen-binding ability after conjugation, and moreover, their different chemical composition compared to the antibody’s peptide chain allows for site-specific modifications, making them suitable conjugation sites.
Enzyme-Guided Modification
Payload attachment can be achieved in a highly selective manner by inserting specific amino acid tags into the antibody sequence. These tags are recognized by specific enzymes, such as formylglycine-generating enzyme (FGE), microbial transglutaminase (MTG), transpeptidase, or tyrosinase, allowing for site-specific conjugation.
Cysteine Engineering: ThioMab Technology
ThioMab technology achieves selective and uniform modification of desired sites on the antibody by utilizing engineered reactive cysteines that do not involve structural disulfide bonds. Generally, the design of cysteine mutations aims to facilitate the conjugation of cytotoxic payloads while maintaining the stability, affinity, and minimizing ADC aggregation of the monoclonal antibody. To determine the optimal positions for mutations, various techniques are typically employed, including computational modeling, model system screening, and high-throughput scanning.
Engineering Non-Natural Amino Acids
In addition to ThioMab technology, the incorporation of non-canonical amino acids (ncAA) provides another possibility for site-specific conjugation. This technology utilizes amino acids with unique chemical structures, allowing for the selective introduction of linkers-payload complexes in a chemical manner. This technology requires the re-engineering of the antibody sequence, using tRNAs and aminoacyl-tRNA synthetases (aaRS) that are orthogonal to all endogenous tRNAs and synthetases within the host cell, to respond to unassigned codons and incorporatencAA into proteins. Typically,ncAA is added to the culture medium during fermentation. Choosing non-natural amino acids is crucial, as they may provoke immunogenicity. Commonly usedncAA are analogs of natural amino acids with unique groups, such as ketones, azides, cyclopropenes, or dienes.
4
Challenges of ADC
Next-generation ADCs with improved strategies can achieve optimal stability, specificity, and relatively low off-target toxicity, thereby enhancing the therapeutic index. Nevertheless, many challenges remain to be addressed, such as pharmacokinetics, targeted specific payload release, uniform distribution of anticancer drugs in tumor areas, adverse side effects, and resistance.
Complex Pharmacokinetic Characteristics
After ADC administration, there may be intact ADCs, naked antibodies, and free forms of cytotoxic payloads present in the serum. In the typical pharmacokinetic characteristics of ADC, the concentrations of conjugated ADCs and naked antibodies continue to decrease with the internalization of ADC and antibody clearance. The two main factors affecting ADC clearance rates are the decoupling of the cytotoxic payload from the antibody and FcRn-mediated recycling.
Free cytotoxic payloads are primarily metabolized in the liver and excreted through the kidneys (urine) or feces, which may lead to liver and kidney dysfunction. All these factors, coupled with significant inter-patient variability, make it challenging to establish PK and PD models to describe the clinical characteristics of ADCs and assist in designing new ADCs.
Payload Release
Treating solid tumors is more complex than hematological cancers. Due to the high molecular weight of ADCs, they have difficulty penetrating tumor sites. Current research indicates that only a small portion of the ADC administered to patients can reach the tumor cells; thus, the potency of the payload needs to be considered when designing ADCs.
Unavoidable Side Effects
The most critical factor associated with unavoidable side effects is the premature release of the payload in systemic circulation; additionally, the antibodies of the ADC may induce immunogenic side effects in the body. Thrombocytopenia, anemia, neutropenia, leukopenia, and hepatotoxicity are the most common toxicities observed clinically. Moreover, in HER-2 specific ADCs, pulmonary toxicity such as interstitial lung disease (ILD) has been observed. Therefore, careful optimization of the next-generation ADCs is necessary to develop ADCs with minimal side effects.
Resistance
Another significant factor that cannot be overlooked is resistance. Current evidence suggests that tumors can develop ADC resistance through various mechanisms, such as lowering antigen expression levels, altering intracellular transport pathways, and developing resistance to the payload.
5
Conclusion
ADC is an emerging cancer therapeutic technology with the potential to overcome the limitations of traditional treatments. However, the pharmacology of ADCs is very complex, and there are still challenges in designing and synthesizing ideal ADCs. Therefore, a deep understanding of the factors affecting ADC efficacy can guide the development of better and more efficient ADCs. By selecting appropriate antigens, antibodies, linkers, payloads, and conjugation technologies, ADC designs with stronger efficacy, safety, and stability can be achieved. With the continuous efforts of researchers in this field, it is not difficult to imagine that future ADCs will present more surprises in targeted cancer therapy.
